


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 13-8-2017
Date: 23-11-2015
Date: 11-9-2017
|
Sulfolane
Sulfolane (tetramethylene sulfone) is produced by the reaction of butadiene and sulfur dioxide followed by hydrogenation:
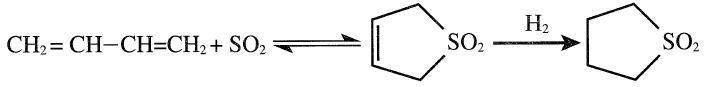
Optimum temperature for highest sulfolene yield is approximately 75°C. At approximately 125°C, sulfolene decomposes to butadiene and SO2. This simple method could be used to separate butadiene from a mixture of C4 olefins because the olefins do not react with SO2. Sulfolane is a water-soluble biodegradable and highly polar compound valued for its solvent properties. Approximately 20 million pounds of sulfolane are consumed annually in applications that include delignification of wood, polymerization and fiber spinning, and electroplating bathes. It is a solvent for selectively extracting aromatics from reformates and coke oven products.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|