


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-4-2017
Date: 18-1-2017
Date: 2-3-2018
|
Buffer Solutions
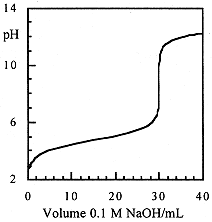
In Figure above, we see that the pH changes most slowly in the vicinity of the half-equivalence point, the buffer region. Small additions of base (or acid) to a solution containing comparable amounts of an acid and its conjugate base change the pH only slightly; the solution is said to be buffered against pH changes. Consider 100 mL of a solution containing 0.1 M CH3COOH and 0.1 M CH3CO2-, pH = 4.75. Addition of 10-3 mol of HCl would change the ratio [CH3CO2-]/[CH3COOH] from 0.1/0.1 to 0.09/0.11 and the pH would change from 4.75 to 4.66. We could, of course, prepare 100 mL of a pH 4.75 solution by diluting HCL to 1.78 × 10-5 M. However, addition of 10-3 mol of HCl would reduce the pH to 2.0, a much bigger change than for the buffer solution.
Example
How many moles of NaH2PO4 and Na2HPO4 should be used to prepare 250 mL of a solution with a total phosphate concentration of 0.10 M and pH = 7.00?
The relevant equilibrium is the second proton transfer step for phosphoric acid,




|
|
|
|
كيف تساهم الأطعمة فائقة المعالجة في تفاقم مرض يصيب الأمعاء؟
|
|
|
|
|
|
|
مشروع ضخم لإنتاج الهيدروجين الأخضر يواجه تأخيرًا جديدًا
|
|
|
|
|
|
|
المجمع العلمي يختتم دورته القرآنية في فن الصوت والنغم بالطريقة المصرية
|
|
|