


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 8-10-2018
Date: 1-7-2019
Date: 23-5-2017
|
Preparation of Alkynes: Elimination Reactions of Dihalides
Alkynes can be prepared by the elimination of HX from alkyl halides in a similar manner as alkenes. Treatment of a 1,2-dihaloalkane (a vicinal dihalide) with an excess amount of a strong base such as KOH or NaNH2 results in a twofold elimination of HX and formation of an alkyne . The starting vicinal dihalides are themselves readily available by addition of Br2 or Cl2 to alkenes. Thus, the overall halogenation/dehydrohalogenation sequence makes it possible to go from an alkene to an alkyne. For example, diphenylethylene is converted into diphenylacetylene by reaction with Br2 and subsequent base treatment.

The twofold dehydrohalogenation takes place through a vinylic halide intermediate, which suggests that vinylic halides themselves should give alkynes when treated with strong base. (Remember: A vinylic substituent is one that is attached to a double-bond carbon.) This is indeed the case. For example:
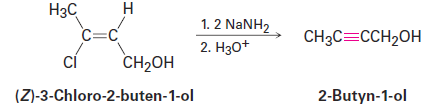



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|