


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 8-8-2016
Date: 14-8-2016
Date: 22-8-2016
|
β-decay of Tritium
Tritium is an isotope of hydrogen with one proton and two neutrons. A hydrogen-like atom is formed with an electron bound to the tritium nucleus. The tritium nucleus undergoes β-decay, and the nucleus changes its charge state suddenly to +2 and becomes an isotope of helium. If the electron is initially in the ground state in the tritium atom, what is the probability that the electron remains in the ground state after the sudden –β-decay?
SOLUTION
We use the sudden approximation to calculate the probability that the electron remains in the ground state. One calculates the overlap integral I of the initial and final wave functions, and its square is the probability. The ground states in the initial and final states are called ѱi and ѱf, and a0 is the Bohr radius:
 (1)
(1)
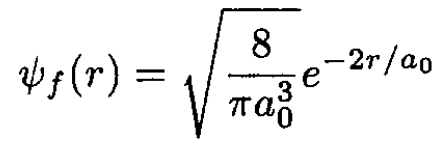 (2)
(2)
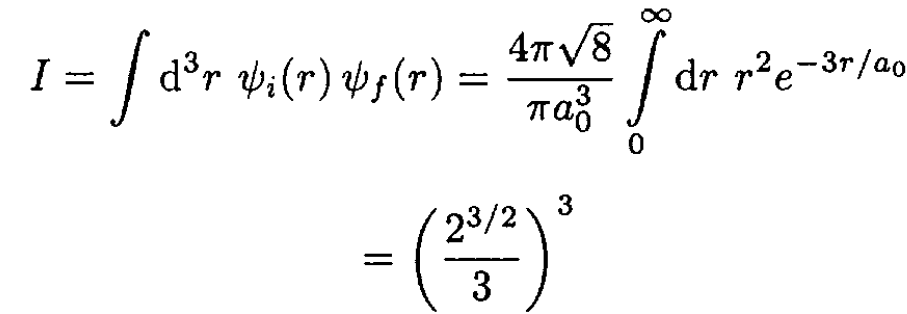 (3)
(3)
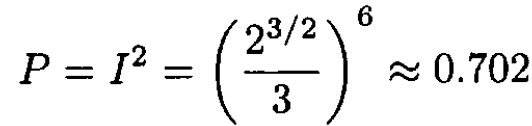 (4)
(4)



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|