


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 20-12-2015
Date: 27-10-2020
Date: 15-9-2019
|
Synthesis of Carbonyl Compounds via Ozonolysis Alkenes
In all the alkene addition reactions we’ve seen thus far, the carbo carbon double bond was converted into a single bond but the carbon skeleton was unchanged. There are, however, powerful oxidizing reagents that will cleave C=C bonds and produce two carbonyl-containing fragments.
Ozone (O3) is perhaps the most useful double-bond cleavage reagent. Prepared by passing a stream of oxygen through a high-voltage electrical discharge, ozone adds rapidly to a C=C bond at low temperature to give a cyclic intermediate called a molozonide. Once formed, the molozonide spontaneously Rearranges to form an ozonide. Although we won’t study the mechanism of this rearrangement in detail, it involves the molozonide coming apart into two fragments that then recombine in a different way.

Low-molecular-weight ozonides are explosive and are therefore not isolated. Instead, the ozonide is immediately treated with a reducing agent, such as zinc metal in acetic acid, to produce carbonyl compounds.
The net result of the ozonolysis/reduction sequence is that the C=C bond is cleaved and an oxygen atom becomes doubly bonded to each of the original alkene carbons. If an alkene with a tetrasubstituted double bond is ozonized, two ketone fragments result; if an alkene with a trisubstituted double bond is ozonized, one ketone and one aldehyde result; and so on.

Several oxidizing reagents other than ozone also cause double-bond cleavage, although such reactions are not often used. For example, potassium permanganate (KMnO4) in neutral or acidic solution cleaves alkenes to give carbonyl-containing products. If hydrogens are present on the double bond, carboxylic acids are produced; if two hydrogens are present on one carbon, CO2 is formed.

In addition to direct cleavage with ozone or KMnO4, an alkene can also be cleaved in a two-step process by initial hydroxylation to a 1,2-diol, as discussed in the previous section, followed by treatment of the diol with periodic acid, HIO4. If the two - OH groups are in an open chain, two carbonyl compounds result. If the two - OH groups are on a ring, a single, open-chain dicarbonyl compound is formed. As indicated in the following examples, the cleavage reaction takes place through a cyclic periodate intermediate.
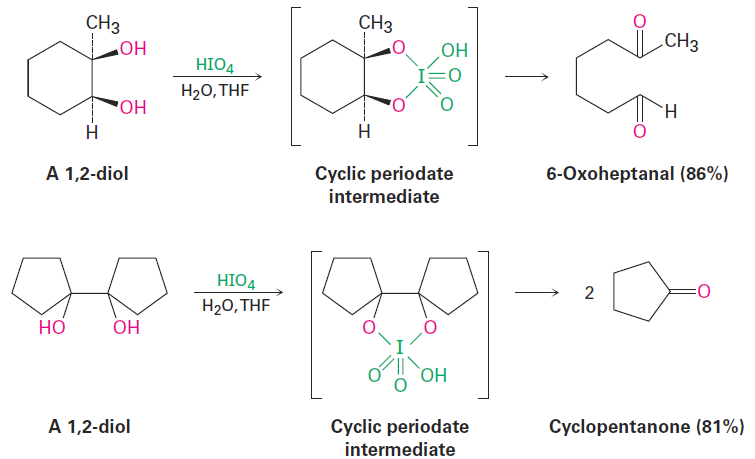



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
قسم شؤون المعارف ووفد من جامعة البصرة يبحثان سبل تعزيز التعاون المشترك
|
|
|