

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Poly(3-hydroxy-butyrate), or PHB, and copolymers
المؤلف:
Faris Yılmaz
المصدر:
POLYMER SCIENCE
الجزء والصفحة:
p28
13-4-2016
1546
Poly(3-hydroxy-butyrate), or PHB, and copolymers
Polyhydroxyalkanoates (PHAs) are polyesters of several hydroxyalkanoates that are synthesized by many microorganisms as a carbon and energy storage material. The hydroxyalkanoates can be synthesized from natural substances such as sucrose (e.g. from sugar cane), carboxylic acids and alcohols. Precisely for this reason, this material is rapidly biodegraded in various environmental conditions by many different organisms. Poly(R-3-hydroxybutyrate) (PHB) is a homopolymer of R-3-hydroxybutyrate, and the best known polymer of the PHA group . The molecular weight generally varies from 50,000 to 2,000,000 g.mol-1. The monomers are all optical isomers R, the only ones capable of being hydrolyzed by depolymerases, in the isotactic form.
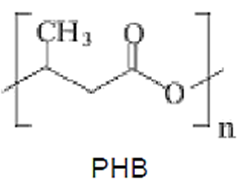
PHAs polymers and copolymers are semicrystalline, with the molecules conformed in helices in the crystalline lamellae, which form spherulites. The native intracellular granules of PHAs with 0.2-0.7 μm in diameter, are amorphous and covered by a protein surface layer of about 2-4 nm, containing phospholipids. PHAs are attacked by intracellular PHAsdepolymerases enzymes, but not extracellular depolymerases.
PHB is a biological storage material that is used by archaea, bacteria and fungi as feed source. There are more than 75 bacterial genera capable to synthesize PHAs, that are also produced by archaea, fungi, plants and animals, in the soils and aquatic bodies. In addition to the well known 3-hydroxybutyrate, more than 100 monomeric constituents have already been identified.
Bacteria and archae can accumulate PHB up to about 95% by dry weight in their cells in the form of granules. The PHB synthesis can occur, for example, as follows: the bacteria are inoculated in a small batch reactor, along with sucrose, other nutrients and water, pH is adjusted and the temperature is raised. The growing colony is transferred to successively larger reactors. After the initial growth of bacteria, the competition period starts, with bacterial storage of PHB in the cytoplasm. The molecular weight increases continuously up to reactor cooling and addition of solvent, what will cause cell lysis and dissolve PHB, which is then purified and dried. The powder obtained in the extraction process is transformed into pellets in an extruder. At the same time nucleating agents and plasticizers are added to improve processability and mechanical properties. There are also successful attempts to develop genetically modified plants to produce PHAs , but the products obtained are very expensive, not being accepted by the market.
As PHB is a very rigid and brittle polymer, it may be mixed with other polymeric materials that are softer and more strainable, such as PBAT or PCL. Blend-stabilizing copolymers, with an intermediate chemical structure, may be obtained by separated synthesis, by transesterification or by the action of peroxides on the two components. A significant product is the copolymer poly(3-hydroxybutyrate-co-3-hydroxy-valerate), or PHBV, which presents lower crystallinity and rigidity than PHB, increasing the flexibility and the elongation capacity.
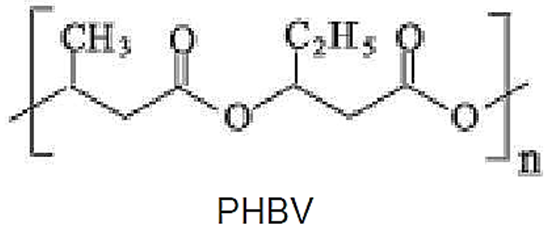
Some important PHAs manufacturers are Metabolix/ADM (Mirel and Mvera, 50 kt/annum), Biomer, Tianan (PHBV, 1 kT/annum, being expanded) and PHB Industrial (60 t/annum pilot plant). As for processing, the material depolymerizes at high temperatures, therefore the temperature of 185 °C should not be exceeded. At 195 °C and up decomposition takes place, with emission of inflammable gases and darkening.
Some applications are: tubes for seedlings, injection and blown moulded containers, and films [for example, obtained with PHBH, or poly (3-hydroxybutyrate-co-3-hydroxy-hexanoate)]. For medical applications, the price of PHAs is already acceptable, although it is still too high for the commodity market, such as for packaging. Some important aspects to be improved in PHB are: strong degradation during processing1, excessive brittleness and low elongation capacity2. The latter can be solved through the use of copolymers and blends. The PHAs may undergo simultaneously hydrolytic, oxidative and enzymatic degradation. The PHAs degrading microorganisms are widely distributed in the environment. Just as bacteria and archaea, fungi are also excellent decomposers.
In addition to their high degradative potential, many fungi have remarkable capacity to expand on the substrate surface, surrounding it with their hyphae, which release extracellular enzymes close enough to achieve the substrate. PHAs are biodegradable in windrow composting, soil or marine sediments. The enzymes which are involved in the degradation of PHAs are depolymerases, hydrolases which may be intra- or extracellular and endo- or exoenzymes3. They cleave the chain into smaller fragments (hydroxy acids), either monomers or oligomers, used as sources of carbon and energy4. The chains must be previously cleaved by extracellular depolymerases, then soluble monomers and oligomers are introduced and metabolized within the cells. Unlike the aquatic environment, the soil environment makes it difficult to transport the enzymes secreted by microorganisms over long distances to the substrate. Biodegradation under aerobic conditions results mostly CO2, H2O and biomass, whereas, in anaerobic conditions, it results mainly, in addition to the above components, CH4 (methane). PHB is abiotically degraded by hydrolysis, with random cleavage of the ester linkages, especially at high temperatures (above 160-170 oC). It is also biotically degraded by many genera of bacteria, archaea and fungi, with biofilm formation. The rate of biodegradation with PHA depolymerase is 102-103 faster than that of hydrolytic degradation. The degradation of the amorphous regions is faster than that of the crystalline regions. In the PHBV copolymer, the crystallinity maintained constant, the addition of 3-hydroxy-valerate decreases the hydrolysis rate, as well as the enzymatic degradation rate. Longer side chains on the β- carbon further reduce the possibility of enzymatic attack.
…………………………………………………………………………
- PHB undergoes β-elimination reactions, which cleave molecules and form chains with terminal unsaturation.
- This is a consequence of the high crystallinity and the large spherulites formed, since the crystals nucleate slowly but grow fast.
- The enzymes may be classified as intra- or extracellular according their action inside or outside the cell, and also as endo- or exoenzymes, according their action inside or at the end of the substrate molecule.
- They are usually induced enzymes whose expression is repressed in the presence of other carbon sources such as glucose and organic acids.
 الاكثر قراءة في كيمياء البوليمرات
الاكثر قراءة في كيمياء البوليمرات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















