

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Counting colonies and calculating results
المؤلف:
SILVA, N.D .; TANIWAKI, M.H. ; JUNQUEIRA, V.C.A.; SILVEIRA, N.F.A. , NASCIMENTO , M.D.D. and GOMES ,R.A.R
المصدر:
MICROBIOLOGICAL EXAMINATION METHODS OF FOOD AND WATE A Laboratory Manual
الجزء والصفحة:
20-3-2016
20211
Counting colonies and calculating results
The instructions contained in this item are applicable to the tests in which all the colonies that developed on the plates, after the incubation period, are counted and considered in any further calculation(s). In the case of tests that use differential media – performed to distinguish the target microorganism(s) from accompanying microbiota (i.e. other microorganisms that may grow under the same conditions) – only the typical colonies are counted and considered in any further calculation(s). This is the case of enterococci and Enterobacteriaceae counts, which should be calculated in accordance with the guidelines provided in specific chapters. In the same way, in the case of tests that require confirmation of the colonies, only the percentage of confirmed colonies is considered in the calculation (s). The latter is the case of lactic acid bacteria, C. perfringens, S. aureus and B. cereus counts, all of which should be calculated following the instructions and guidelines provided in specific chapters.
1- Pour plate calculations
Select for counting plates without spreading and with a number of colonies in the range between 25 and 250. Count the colonies with the help of the magnifying glass of a colony counter to facilitate visualization. Use a colony counter with a 1 cm2 square grid background to serve as a counting guide. If no such plates in ideal conditions are available, follow the instructions described in rules 5 through 12 for counting.
To calculate the results, two situations are to be considered. The first is the standard situation and the second is the samples prepared by the surface swabbing or surface washing techniques.
A- Calculating the pour plate results in the standard situation
The standard situation is that in which the analytical unit consists of a mass (weight) or volume of the sample, homogenized with the diluent. The general rule for calculating the results is: CFU/g or CFU/ml = c/d.v, where c is the number of colonies on the counted plate, d the dilution rate of the counted plate and v the inoculated volume of this dilution. More detailed rules for calculating the results follow below.
Rule 1 – If the count was performed on a plate inoculated with an undiluted sample, without duplicate, the number of colony forming units (CFU) is equal to the number of colonies. If a duplicate was made, the number of CFU is equal to the arithmetic average of the counts obtained in each of the plates of the duplicate.
Rule 2 – If the count was performed on a plate inoculated with a 10−1 dilution or greater, without duplicate, calculate the number of CFU/g or ml by multiplying the number of colonies by the inverse of the inoculated dilution. The inverse of the 10−1 dilution is 101, the inverse of the 10−2 dilution is 102 and so forth. If a duplicate was made, consider as the number of colonies the arithmetic average of the counts obtained in each of the plates of the duplicate and multiply by the inverse of the dilution.
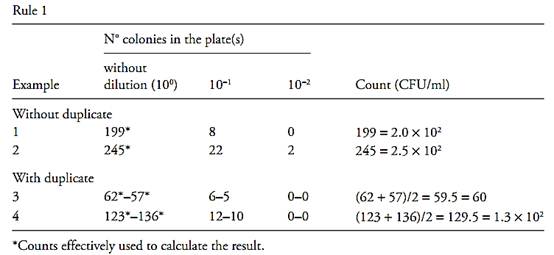

Rule 3 – If the inoculated volume of the first dilution (or of the sample without dilution) is different from 1 ml and the count was performed on the plate inoculated with this volume, rules 2 and 3 apply, but the number of colonies must be divided by the inoculated volume in order to calculate the result.
Rule 4 – If the initial dilution is not decimal, 1:20, 1:50, 1:200), or other), rules 2 and 3 apply, but it is necessary to insert into the calculations the actual initial dilution used. Considering an analytical unit of m grams or milliliters, diluted in v milliliters of diluent, the initial dilution will be equal to m/(m+v), that is, the analytical unit divided by the total volume (diluent + analytical unit). The subsequent decimal dilutions will be the initial dilution multiplied by 10−1 (1st decimal), the initial dilution multiplied by 10−2 (2nd decimal) and so forth. For example, for an analytical unit of 50 g pre-pared with 950 ml of diluent, the initial dilution is 50/(50 + 950) = 50/1.000 = 1/20 (1:20). The 1st decimal is 10−1/20, the 2nd decimal is 10−2/20 and so forth. The results can also be calculated by multiplying the number of colonies by the inverse of the dilution, but in this case, the inverse of the dilution is the inverted fraction: the inverse of a 1/20 dilution = 20/1, the inverse of a 10−1/20 dilution = 20 × 101, the inverse of a 10−2/20 dilution = 20 × 102 and so forth (Examples 13, 14, 15, 16, 17 and 18).
Rule 5 – One duplicate plate with counts above or below the range of 25–250 colonies. If the other plate exhibits counts in the 25 to 250 range, the number of both plates must be considered when calculating the result .
Rule 6 – Two consecutive dilutions with 25–250 colonies. Calculate the number of CFU of each dilution and compare the results.
6a) If one of the results is greater than the double of the other, consider only the lower count.
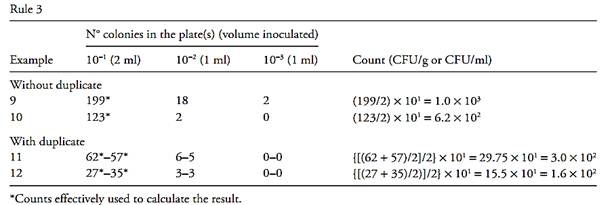

Table.1 Examples for calculating the pour plate results in not ideal conditions.
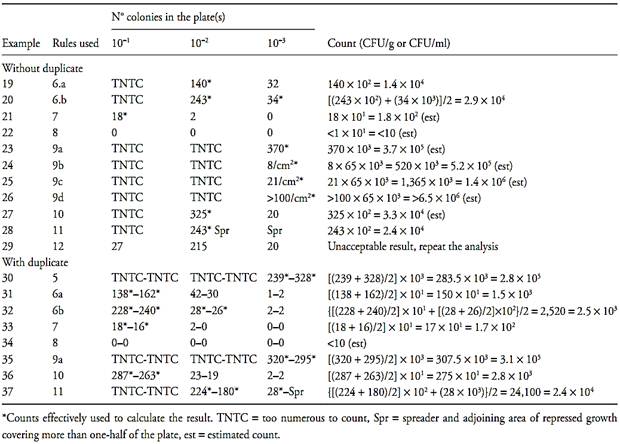
6b) If one of the results does not exceed the double of the other, then both results must be considered, and the mean value should be presented as the final result (Examples 20 and 32 of Tabl.1).
Rule 7 – None of the plates reached 25 colonies. Count the plates exhibiting a number of colonies closest to 25, calculate CFU number (Examples 21 and 33 of Table.1) and report the result as estimated count (est).
Rule 8 – No plate showing growth. Consider the number of colonies of the 1st inoculated dilution as being one and calculate the result in accordance with rules 1, 2, 3 or 4 (Examples 22 and 34 of Table.1). Report the final result as being smaller than the value obtained by the calculation, estimated value.
Rule 9 – All plates containing more than 250 colonies. In these cases, there are four alternatives for estimating the number of CFU/g or ml. In all cases, the result must be reported as estimated count (est).
9a) If it is possible to count all the colonies on the plate, count and calculate the number of CFU from the counts obtained (Examples 23 and 35 of Table.1).
9b) If it is not possible to count all colonies on the plate, but the number of colonies per cm2 is lower than 10, count the colonies in 12 of the 1 cm2 squares, six consecutive squares in a row and six consecutive squares in a column, using the squares traced on the grid background of the colony counter as counting guide. Calculate the average number of colonies/cm2 and use this average value to determine the total number of colonies on the plate by multiplying the average value by the total surface area of the plate. Remember that the total surface area of the plate is equal to πd2/4, where d is the inner diameter. For example, 100 mm-plates have an inner diameter of about 9 cm and a total surface area of 65 cm2. Use the total number of colonies thus calculated to determine the number of CFU (Example 24 of Table.1).
9c) If the number of colonies per cm2 is greater than 10, count the colonies in four squares representative of the distribution of the colonies on the plates and calculate the number of CFU in the same way as described for the case of 12 squares (Example 25 of Table.1).
9d) If the number of colonies per cm2 is greater than 100, report the result as being greater than the total surface area of the plate × inverse of the dilution (Example 26 of Table.1).
Rule 10 – Number of colonies greater than 250 in one dilution and lower than 25 in the next. If in a dilution the number of colonies was higher than 250 and in the next dilution the number of colonies was below this number, select the plates with the counts closest to 250 and calculate the number of CFU from the count obtained (Examples 27 and 36 of Table.1).
Rule 11 – Plates with spreading. There are two types of spreading. The first type results from the disintegration of cell clusters or groupings which may occur when mixing the inoculum with the culture medium. The second type is the result of inadequate mixing of the inoculum with the medium, leading to the forma-tion of thin films of moisture either onto the surface of the medium or between the medium and the bottom
of the plate. The difference between the two types is visually distinguishable, since in the case of spreading of the first type the growth of individual colonies can be observed, whereas in the other case, the growth of the cell mass is continuous, without individual colonies.
Plates displaying spreading can be counted under the following conditions: if none of the individual spreading zones is of a size exceeding 25% of the surface area of the plate, and, also, if the total surface area covered with spreading zones does not surpass 50% of the plate. If these two conditions are not met, report the result as a “laboratory accident” and repeat the test. If the laboratory observes the occurrence of spreading of the second type, with spreading zones consistently greater than 25% of the total plate surface, in more than 5% of the plates prepared within a certain period of work time, preventive measures should be taken to minimize this problem. To count plates with spreading zones of the first type, each zone should be counted as one sin-gle CFU, and the individual colonies within each of these zones should not be counted. To count plates dis-playing spreading zones of the second type, select one region of the plate, free of spreading and count the col-onies within several of the 1 cm2 squares. Calculate the average of the colonies per cm2, multiply by the total surface area of the plate (65 cm2 in the case of plates with an external diameter of 100 mm) and use this esti-mated value to calculate the number of CFU. Report the result as estimated count (est) (Examples 28 and 37 of Table 1).
Rule 12 – Plates in which microbial growth is proportionally greater in the greatest dilutions. This situation may occur as a result of accidental contami-nation of the sample during plating, incorrect identification of the sample dilution rate on the plates or be caused by the presence of inhibitory substances in the sample. Consider the result as a “laboratory accident” and repeat the test. If the suspicion of the presence of inhibitory substances in the sample is high, repeat the test using an adequate procedure to eliminate or reduce the influence of these components on the result (Exam-ple 29 of Table 1).
B- Calculating the pour plates results for samples prepared by the surface swabbing technique (swabs or sponges)
The results should be expressed in CFU/cm2 of sample. Initially it is necessary to calculate the number of CFU per milliliter of the diluent in which the swabs were placed prior to analysis. For that purpose, consider this suspension as a non-diluted sample and, as a function of the dilutions inoculated of this suspension, calculate the result in exactly the same way as described for the standard situation .Next, the CFU/ml count of the suspension should be converted to CFU/cm2 of the sample. To that purpose, calculate to how many cm2‘s each milliliter of the suspension corresponds. a surface area of 50 cm2 is sampled and the swabs placed in 10 ml diluent, with each milliliter of diluent corresponding to 5 cm2 of the sampled surface. This ratio, however, may be changed at the discretion of the laboratory, depending on the type of sample and the objective of sampling.
It is recommendable to work always with diluent volumes that are a multiple of the sampled areas to facilitate calculations. In the case above, the CFU/cm2 count will be equal to the value obtained per ml of the suspension, divided by five. for sponge sampling, a surface area of 100 cm2 is sampled and the sponges placed in 25 ml diluent, with each milliliter of the diluent corresponding to 4 cm2 of the sampled surface. In this case, the CFU/cm2 count will be equal to the value obtained per ml of the suspension, divided by four. In another situation, in which a swab-bing suspension yielded by swabbing a surface area of 100 cm2 were to be suspended in 10 ml of diluent, for example, each ml of the suspension would correspond to 10 cm2 of the area and the number of CFU/cm2 would be equal to the value obtained per ml of the suspension, divided by ten.

C- Calculating the pour plate results for samples prepared by the surface washing technique
In the case of foods, the results should be expressed in CFU/g of sample. Initially, the number of CFU should be calculated per milliliter of washing diluent. For that purpose, consider this washing suspension as a non-diluted sample and, as a function of the dilutions inoculated of this suspension, calculate the result in exactly the same way as described for the standard situation . Next, the CFU/ml count of the washing suspension should be converted to CFU/g of sample, as a function of the initial dilution used to perform the washing procedure (sample weight: diluent volume). If the dilution was 1:1 each ml of the washing suspension will correspond to 1 g of sample and the number of CFU/g will be equal to the value obtained per ml. If the dilution is different from 1:1, first it is necessary to calculate to how many grams of sample each 1 ml of the washing suspension corresponds, which is equal to the weight of the washed sample divided by the volume of diluent used. For exam-ple, if a chicken carcass weighing 1.6 kg was washed with 400 ml of diluent, each ml of washing diluent will correspond to 4 g of the sample. In this case, the number of CFU/g sample will be equal to the number of CFU/ml of washing diluent, divided by four.

In the case of packages, the results may be expressed in CFU/cm3 of the package or in CFU per package. Initially, the number of CFU per milliliter of washing water should be calculated, in exactly the same way as indicated for foods.
Next, the CFU/ml number of the washing water should be converted to CFU/cm3, as a function of the volume of diluent used to perform the washing procedure. For that purpose, first it is necessary to calculate to how many cm3 of the package each 1 ml of the washing water corresponds. This value is equal to the holding capacity of the package divided by the volume of the diluent used. For example, if a package with a hold-ing capacity of 500 ml was washed with 100 ml dilu-ent, each milliliter of washing water will corresponds to 5 cm3. In this example the number of CFU/cm3 would be equal to the number of CFU/ml of the washing water divided by five.

To determine the number of CFU per package, it suffices to multiply the number of CFU/cm3 by the holding capacity of the package.

2- Spread plate calculations
As in the case of pour plating, the recommendations presented in this item are applicable to the tests in which all the colonies that have developed on the plates, after completion of the incubation period, are counted and considered for performing the necessary calculations. The colony counts and calculation of the results are done in exactly the same way as described for pour plate, and following the same rules. However, the final result must be multiplied by 10 (ten), to account for the 10-fold smaller volume inoculated. If 1 ml of the first dilution was distributed over several plates, then the number of colonies of this dilution is the sum total of all the plates. If the result is calculated based on the count of this dilu-tion, then it is not necessary to multiply by 10.
3- plate calculations
As in the case of pour plating, the recommendations presented in this item are applicable to the tests in which all the colonies that have developed on the plates, after completion of the incubation period, are counted and considered for performing the necessary calculations. Count the colonies of the drops that contain at most 30 colonies. To calculate the number of CFU/g or ml, take the average of the number of colonies in the three drops of the inoculated dilution, multiply by the inverse of the dilution and then by 100, to account for the inoculated volume (CFU/g or ml = N° colonies × 100/dilution).
4- Membrane filtration calculations
As in the case of pour plating, the recommendations presented in this item are applicable to the tests in which all the colonies that have developed on the plates, after completion of the incubation period, are counted and considered for performing the necessary calculations.
Begin counting the colonies with the aid of a stereoscopic microscope at a magnification of 10 to 15 times and, to facilitate visualization, position the plates so as to obtain illumination perpendicular to the plane of the membrane. Select for the counting only the plates containing 20 to 200 colonies. Follow the rules below to count and calculate the number of CFU/ml:
Rule 1 – If the number of colonies per square of the membrane is smaller than or equal to two, count all the colonies present and divide by the filtered volume to obtain the number of CFU/ml.
Rule 2 – If the number of colonies per square of the membrane is in the range between three and ten, count ten small squares and take the average per square. Multiply this value by 100 and divide by the volume filtered to obtain the number of CFU/ml. If the number of colonies per square falls within the 10 to 20 range, count only 5 squares to take the average and calculate the number of CFU/ml in the same way.
Rule 3 – If the number of colonies per square is greater than 20, express the result as greater than 2000 divided by the filtered volume.
Rule 4 – If filtration was performed on a solution obtained from a solid sample (salt or sugar, for example), rules one, two, and three apply, though the value must be converted to CFU/g as a function of the amount of sample contained in the solution. Example: 25 g of sugar are dissolved in 225 ml of 0.1% peptone water (1:10 dilution); 100 ml of this solution are filtered and 120 colonies are counted on the membrane. Each milliliter of the solution equals 0.1 g of the sample, thus, 100 ml of the filtered solution contains the equivalent of 10 g of the solid sample. Hence: CFU/100 ml solution = CFU/10 g sample = 120 ⇒ CFU/g = 120/10 = 12
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















