


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 19-2-2016
Date: 2025-03-23
Date: 21-2-2016
|
Mendelian disorders
- Each mendelian disorder is caused by a single mutant gene.
→ affects transcription, mRNA processing, or translation
→ abnormal protein or decreased protein
→ may affect any type of protein →Disease.
- show the classic mendelian patterns of inheritance.
- are also called monogenic mendelian disorders.
- are uncommon.
- can be classified into the following based on their patterns of inheritance:
1. Autosomal dominant inheritance
2. Autosomal recessive inheritance
3. X-linked recessive inheritance
The mode of inheritance for a given phenotypic trait/disease is determined by pedigree analysis in which all affected & unaffected individuals in the family are recorded in a pedigree using standard symbols & indicating the sex, the generation, & biologic relationship among the family members. In all mendelian disorders, the distribution of the parental alleles to their offspring depends on the combination of the alleles present in the parents.
Autosomal dominant disorders
- will be discussed under the following 4 headings:-
a. The criteria for autosomal inheritance
b. Additional features of autosomal dominant disorders
c. Pathogenesis
d. Clinical examples
- Dominant implies that the disease allele needs to be present only in a single copy (as in the heterozygote) to result in the phenotype.
a. The criteria of autosomal inheritance include:
i. The transmission of the trait is from generation to generation without skipping. In a typical dominant pedigree, there can be many affected family members in each generation.
ii. Except for new mutation, every affected child will have an affected parent Some patients do not have affected parents because the disease in such cases is due to new mutations in the sperm/ovum from which the patients were derived. New germ line mutations occur more frequently in fathers of advanced age.
Iii. In the mating of an affected heterozygote to a normal homozygote (the usual situation), each child has a 50% chance to inherit the abnormal allele & be affected & a 50 % chance inherit the normal allele. See Fig.1 below.
iv. The 2 sexes are affected in equal numbers (because the defective gene resides on one of the 22 autosomes (i.e. nonsex chromosomes). The exceptions to this rule are the sex-limited disorders such as breast & ovarian cancers in females & familial male precocious puberty in boys.
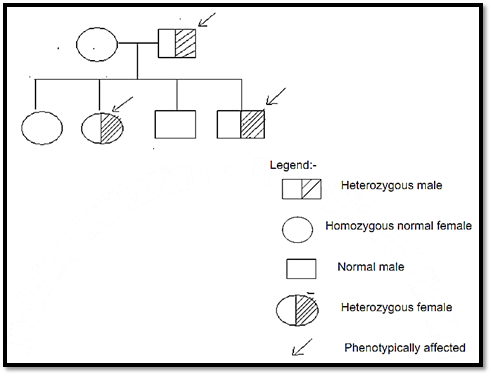
Fig 1. The pedigree for autosomal dominant pattern of inheritance. This figure shows the pedigree for a normal female parent & an affected male parent & their four children. Vertical distribution of the condition through successive generations occurs when the trait does not impair reproductive capacity.
b. Additional features of autosomal dominant disorders
Each of the following may alter the idealized dominant pedigree (& they should be considered to provide the most accurate counselling):-
i. Autosomal dominant disorders can sometimes be caused by new mutations. New mutations may give rise to an isolated case of a dominant disorder. New mutations are more often seen with diseases that are so severe that people who are affected by them are less likely to reproduce than normal. For example, the majority of cases of achondroplasia are the results of new mutations.
ii. Autosomal dominant disorders can show reduced penetrance (i.e. some individuals inherit the mutant gene but are phenotypically normal). Penetrance is the probability of expressing the phenotype given a defined genotype. Penetrance is expressed as the percentage of individuals who have the mutant allele & are actually phenotypically affected. For example, 25% penetrance indicates that 25% of those who have the gene express the trait. Penetrance can be complete or incomplete. Reduced (incomplete) penetrance is when the frequency of expression of a genotype is < 100%. Nonpenetrance is the situation in which the mutant allele is inherited but not expressed.
iii. Autosomal dominant disorders commonly show variable expressivity. Variable expressivity is the ability of the same genetic mutation to cause a phenotypic spectrum. It is when the trait is seen in all individuals carrying the mutant gene but is expressed differently among individuals. For example, some patients with neurofibromatosis type 1 (which is an autosomal dominant disorder) have only brownish spots (café au lait spots) on their skin whereas other patients with the same disease have multiple skin tumors & skeletal deformities. Therefore, neurofibromatosis is said to show variable expressivity. Variable expressivity most likely results from the effects of other genes or environmental factors that modify the phenotypic expression of the mutant allele. For example, individuals with familial hypercholesterolemia who take cholesterol-rich diet have a higher risk of manifesting with atherosclerosis than those individuals with hypercholesterolemia & who take low cholesterol diet. Hence, the variable expressivity in this case is brought about by the influence of an environmental factor (i.e. the diet).In general, variable expressivity & reduced penetrance can modify the clinical picture of autosomal dominant disorders.
c. Pathogenesis of autosomal dominant disorders
Autosomal dominant disorders are caused by 2 types of mutations:
1. Loss of function mutations
2. Gain of function mutations
1. Loss of function mutations cause autosomal dominant disorders when they result in inactive or decreased amount of regulatory proteins (e.g. cell membrane receptors such as LDL receptor), or structural proteins (e.g. collagen, fibrillin, spectrin, dystrophin).
A 50% reduction in the levels of such nonenzyme proteins results in an abnormal phenotype (i.e. the heterozygote, who produces this much amount, will manifest the disorder). This can sometimes be explained by the dominant negative effect of the mutant allele (i.e. product of the mutant allele impairs the function of the product of the normal allele).
2. Gain of function mutations are much less common than loss of function mutations. In such cases, the mutant gene produces a toxic protein (i.e. the protein will have a new toxic function). This is exemplified by Huntington disease. Gain of function mutations almost always have autosomal dominant pattern.
d. Clinical examples of autosomal dominant disorders:
- Marfan syndrome*
- Some variants of Ehlers – Danlos syndrome
- Osteogenesis imperfecta
- Achondroplasia
- Huntington disease
- Neurofibromatosis*
- Tuberous sclerosis
- Myotonic dystrophy
- Familial hypercholesterolemia*
- Hereditary spherocytosis
- Familial polyposis coli
- Polycystic kidney disease
* Only these are briefly described here.
Marfan syndrome
- is a defect of connective tissue characterized by faulty scaffolding.
- is caused by mutations of FBN1 gene → Abnormal fibrillin (which is a structural protein) No normal microfibrils in the extracellular matrix→ No scaffolding on which tropoelastin is deposited to form elastic fibers → Marfan’s syndrome. Microfibrils are normally abundant in the aorta, ligaments, & ciliary zonules of the lens where they support the lens. Hence, Marfan syndrome (in which there is deficiency of normal fibrillin & microfibrils) mainly involves these tissues.
- is characterized by defects in skeletal, visual, & cardiovascular structures:-
i. Patients are tall & thin with abnormally long legs & arms, spider like fingers (arachnodactyly), hyperextensible joints.
ii. Dislocation of the ocular lens (Ectopia lentis) is frequent.
iii. Cardiovascular changes include:
a. Mitral valve prolapse due to loss of connective tissue support in the
mitral valve leaflets.
b. Dilatation of the ascending aorta due to cystic medionecrosis (→lack of medial support).Dilatation of the aortic valve ring & the root of the aorta → Aortic regurgitation.
c. Dissecting aneurysm of the aorta due to medial necrosis & intimal tear.
Familial hypercholesterolemia
- is possibly the most frequent mendelian disorder
- is caused by mutation of the gene for LDL (low density lipoprotein) receptor → Decreased functional LDL receptor → Increased plasma cholesterol → Premature atherosclerosis → Increased risk of myocardial infarction & other complications of atherosclerosis/ The occurrence of xanthomas (which are raised yellow lesions filled with lipid-laden macrophages in the skin & tendons).
- is best understood by knowing the normal process of cholesterol metabolism & transport which is briefly described below.7% of the body’s cholesterol circulates in the plasma, predominantly in the form of LDL. The level of plasma cholesterol is influenced by its synthesis & catabolism. The liver plays an important role in both these processes. The following flow chart illustrates the normal cholesterol metabolism. Abbreviations used in this flow chart
- TG = Triglyceride VLDL = Very Low Density Lipoprotein = has a lot of triglyceride (TG), very little cholesterol, & 3 apoproteins. IDL = Intermediate Density Lipoprotein= has less TG & more cholesterol than VLDL & also has 2 apoproteins.LDL = Low Density Lipoprotein = has much more cholesterol than IDL.
Liver cell Secrets ↓ VLDL ↓
VLDL is transported to the capillaries of adipose tissue or muscle which contain lipoprotein lipase. The lipoprotein lipase degrades the VLDL into TG & IDL.
a. The TG is stored in fat cells or is used for energy in skeletal muscle.
b. The IDL follows 2 pathways:-
i. 50% of plasma IDL is cleared by the liver. The liver uses LDL receptors to remove plasma IDL.
ii. The rest of IDL is converted to plasma LDL (which is cholesterol-rich).↓Plasma LDL is removed by the following 2 pathways:-
1. The scavenger receptor pathway:- In which oxidized LDL or acetylated LDL is removed by a scavenger receptor on the cells of the mononuclear phagocyte system, &
2. Hepatic clearance:- 70% of plasma LDL is removed by the liver (because LDL binds with LDL receptors which are concentrated in certain regions ( called the coated pits) of the cell membrane of the hepatocyte). Then, the coated vesicles containing the bound LDL fuse with the lysosomes.↓ in the lysosomes, LDL is degraded into free cholesterol which enters the cytoplasm.
There, cholesterol does the following things:-
i.It is used for the synthesis of cell membrane & bile acids.
ii. It stimulates storage of excess cholesterol
iii. It inhibits the synthesis of LDL receptors thus protects the cell from excessive accumulation of cholesterol.
Familial hypercholesterolemia
- is caused by different types of mutations in the gene for LDL receptor → No functional LDL receptor → Leads to:-
i. Impaired plasma LDL clearance. This leads to the accumulation of LDL in plasma (i.e. hypercholesterolemia).
ii. Impaired IDL uptake by the liver ( because IDL uses hepatic LDL receptors for this uptake).→ Diversion of a greater proportion of plasma IDL into the precursor pool for plasma LDL. → Hypercholesterolemia.
iii. Increased scavenger receptor – mediated clearance of LDL into the cells of the mononuclear phagocyte system & possibly the vascular walls. This leads to xanthomas & contributes to premature atherosclerosis.
The hypercholesterolemia & the accumulation of LDL inside macrophages produced by the above mechanisms lead to premature atherosclerosis & xanthomas. This knowledge of the pathogenesis of familial hypercholesterolemia has led to a logical discovery of its treatment. We have said that the basic problem in this disease is absence of LDL receptors. Hence, the logical treatment is to increase the number of LDL receptors. (i.e. to remove the basic problem). This can be done by:-
1. Statins
- are drugs which inhibit hepatic HMG CoA reductase→ Inhibits intracellular cholesterol synthesis→ leads to greater synthesis of LDL receptors (See the normal cholesterol metabolism above)
2. Gene therapy (under investigation)
- by giving normal LDL receptor genes via a viral vector.
This illustrates that knowing the pathogenesis of diseases greatly helps not only in understanding their morphologic & clinical features but also in the logical discovery of their treatment.
Neurofibromatosis:-
- is a familial neoplasm. Familial neoplasms have neoplasm-causing mutations transmitted through the germ line. Familial neoplasms account for about 5% of all cancers & they are mendelian disorders. They are often inherited in autosomal dominant pattern with few exceptions. They are caused by mutations that affect proteins which regulate cell growth. And they are exemplified by neurofibromatosis types 1 & 2. It should be noted that most cancers are not familial & these non-familial cancers are caused by mutations of tumor-suppressor genes, proto-oncogenes, & apoptosis- regulating genes in somatic cells. Hence, these mutations are not passed in the germ line. Therefore, most cancers are not mendelian disorders i.e. they are sporadic or nonfamilial disorders. Here, neurofibromatosis which is a mendelian neoplasm is discussed.
1. Neurofibromatosis type 1
- was previously called von Recklinghausen disease.
- has autosomal dominant transmission in 50% of cases. (The rest 50% are due to new mutations.
- has extremely variable expressivity but the penetrance is 100%.
- is due to a mutation in the NF1 gene (which is a tumor-suppressor gene).
- mainly shows neurofibromas in the skin & other locations, café au lait spots (i.e. light brown skin pigmentations), & pigmented iris hamartomas (Lisch nodules). The benign neurofibromas can sometimes become malignant.
- may also show skeletal disorders such as scoliosis & bone cysts & increased incidence of other tumors especially pheochromocytoma & malignancies such as Wilm’s tumor, rhabdomyosarcoma, & leukaemia.
2. Neurofibromatosis type 2
- was in the past called acoustic neurofibromatosis.
- is much less common than neurofibromatosis type 1.
- is an autosomal dominant disorder.
- is due to a mutation of the NF-2 gene ( which is a tumor suppressor gene)
- shows bilateral acoustic schwannomas, multiple meningiomas, & gliomas (typically ependymomas of the spinal cord).
Autosomal recessive disorders
- will be discussed under the following headings:-
a. Criteria
b. Additional features
c. Pathogenesis
d. Clinical examples
In autosomal recessive disorders, the phenotype is usually observed only in the homozygote. The typical pedigree shows affected male & female siblings with normal parents & offspring. Recessive inheritance is suspected when parents are consanguineous; it is considered proven when the corresponding enzyme levels are low or absent in affected individuals & are at half normal values in both parents.
a. Criteria
i. If the trait is rare, parents & relatives other than siblings are usually normal ii. In the mating of 2 phenotypically normal heterozygotes, the segregation frequency with each pregnancy is 25% homozygous normal, 50% heterozygous normal, & 25% homozygous affected. See Fig. 2 below.
iii. All children of two affected parents are affected.
iv. Both sexes are affected in equal numbers
v. If the trait is rare in the population, the probability of parenta consanguinity is increased.
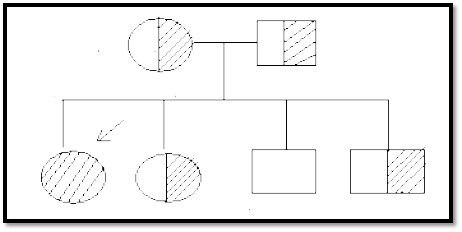
Fig 2. Autosomal recessive pattern. This diagram shows the pedigree for 2 heterozygous parents & their 4 children. Only siblings are affected. Vertical distribution of affected individuals is not usually seen. Horizontal distribution & consanguinity are seen in a multiplex pedigree. (Note: This is not a multiplex pedigree).
b. Additional features
i. Autosomal recessive disorders show more uniform expression of the trait than autosomal dominant disorders. (i.e. they don’t show variable expressivity).
ii. They commonly show complete penetrance.
iii. They frequently show signs & symptoms early in life, whereas many autosomal dominant disorders have delayed onset e.g. Huntington disease clinically manifests for the first time during adulthood.
c. Pathogenesis
Many autosomal recessive disorders are caused by loss of function mutations which result in decreased enzyme proteins.
Homozygotes → No normal enzyme → Disease.
Heterozygotes →Equal amounts of normal & defective enzymes→Cells with half the normal amount of the enzyme function normally →No disease.
d. Clinical examples include:-
- Sickle cell anemia
- Thalassemias
- Congenital adrenal hyperplasia
- Cystic fibrosis Wilson disease
- HemochromatosisMendelian disorders associated with enzyme defects:*
o Phenylketonuria
o Galactosemia
o Homocystinuria
o Lysosomal storage diseases
o Alpha 1 antitrypsin deficiency
o Glycogen storage disease
* These will be discussed further.
- Mendelian disorders associated with enzyme defects.
- have mutations → decreased amount of a normal enzyme or abnormal enzyme with decreased activity→ Metabolic block → Consequences → Disease.
- show autosomal recessive pattern of inheritance because half the normal amount of enzyme is enough for normal function. Hence, heterozygotes (who produce this amount) do not manifest the disease. I.e. the inheritance is autosomal recessive.
- is illustrated by the following model of a metabolic pathway:- Substrate ↓ Enzyme 1↓ Intermediate 1↓ Enzyme 2↓Intermediate 2 →→ M1 →→ M2 ↓ (Minor pathway products) Enzyme 3 ↓ Final product If an enzyme of the above pathway is defective, then the consequences may be:
1. Accumulation of the substrate, &/or one or both of the intermediates, & the products of the minor pathway depending on the level of the block. These substances may be toxic in high concentrations & result in tissue damage. This mechanism occurs in the following diseases:
• Lysosomal storage diseases
• Galactosemia
• Phenylketonuria
2. Decreased amount of the final end product.
• This is exemplified by albinism.
3. Failure to inactivate a toxic substrate. E.g. Hereditary alpha -1 antitrypsin deficiency.
Mendelian disorders associated with enzyme defects include most inborn errors of metabolism such as:
o Lysosomal storage diseases (E.g. Gaucher disease)
o Phenylketonuria
o Severe combined immunodeficiency disease
o Alpha 1 antitrypsin deficiency
o Albinism
o Lesch – Nyhan syndrome
In order to illustrate the basic principles of this category, only the first two disorders from the above list are discussed below in moderate depth.
1. Lysosomal storage diseases
- result from lack of any protein essential for the normal function of lysosomes.
Lysosomes are intracellular organelles used for degrading a variety of complex substrates. They do so by means of a variety of enzymes. The following figure compares the normal lysosomal degradation pathway with that of lysosomal storage disease. Complex substrate
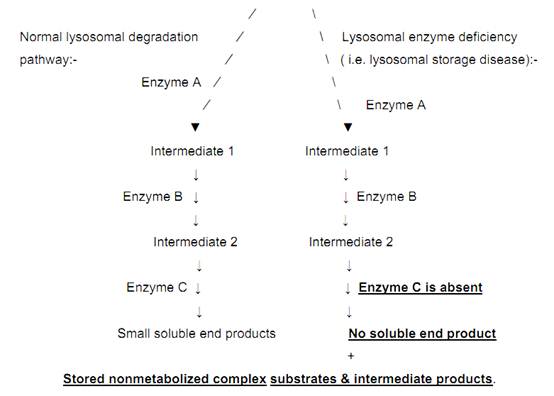
Fig. 3. Normal lysosomal degradation Vs lysosomal storage diseases.
Lysosomal storage diseases can be divided into the following subgroups based on the nature of the accumulated substance:
a. Sphingolipidoses
e.g. Tay-Sachs disease in which there is deficiency of the alpha subunit of the enzyme –hexosaminidase leading to the accumulation of GM2 gangliosides.
b. Sulfatidoses e.g. 1. Gaucher disease e.g. 2. Niemann-Pick disease types A & B (have deficiency of sphingomyelinase resulting in the accumulation of sphingomyelin).
c. Muopolysacharidoses (MPS)
d. Mucolipidoses (ML)
e. Type 2 glycogenosis ( Pompe disease)
f. etc…
The organs affected in lysosomal storage diseases (i.e. the distribution of the stored material) are determined by the following 2 factors:
i. The site where most of the material to be degraded is found.
E.g.1. Brain is rich in gangliosides, hence defective degradation of gangliosides as in Tay-Sachs disease results in the storage of gangliosides within neurons leading to neurologic symptoms. E.g.2. Mucopolysaccharides are widely distributed in the body. Hence, mucopolysacharidoses (i.e. defects in the degradation of polysaccharides) affect virtually any organ.
ii. The location where most of the degradation normally occurs.
Organs rich in phagocytic cells such as the spleen & liver are frequently enlarged in several forms of lysosomal storage diseases. This is because cells of the mononuclear phagocytic system are rich in lysosomes & are involved in the degradation of a variety of substrates.
From among the various types of lysosomal storage diseases listed above, only Gaucher disease is discussed here to illustrate the basic principles of lysosomal storage diseases.
Gaucher disease
- is the most common lysosomal storage disorder.
- is a disorder of lipid metabolism caused by mutations in the gene encoding glucocerebrosidase.→ Deficiency of glucocerebrosidase→ Accumulation of glucocerebroside mainly in the cells of the mononuclear phagocyte system & sometimes in the central nervous system. Glucocerebrosides are continually formed from the catabolism of glycolipids derived mainly from the cell membranes of old red blood cells & white blood cells.
- Morphologically shows Gaucher cells (distended phagocytic cells with a distinctive wrinkled tissue paper cytoplasmic appearance).
- - has 3 clinical subtypes : Type I (chronic nonneuronopathic), Type II ( acute neuropathic) , & Type III (Juvenile).
Type I (Chronic non-neuronopathic form) (Adult Gaucher disease):-
- accounts for 99% of the cases.
- is found mainly in European Jews.
- does not involve the brain.
- shows accumulation of glucocerebrosides only in the cells of the mononuclear phagocytic system throughout the body.
- Hence, it shows Gaucher cells in the spleen, liver, lymph nodes, & bone marrow.
- clinically manifests by
o Splenomegaly→Hypersplenism→Pancytopenia.
o Hepatomegaly
o Generalized lymphadenopathy
o Pathologic fractures & bone pain due to erosion of the bone.
o First appearance of sings & symptoms in adult life.
o Progressive disease which is compatible with long life.
Phenylketonuria (PKU)
- is caused by mutation of the phenylalanine hydroxylase gene → Phenylalanine hydroxylase deficiency → Failure of conversion of phenylalanine to tyrosine in the liver →High serum concentration of phenylalanine which is neurotoxic →Progressive cerebralmyelination
In addition the minor pathways of phenylalanine metabolism produce phenyl pyruvic acid (“phenylketone”) & phenyl acetic acid which are excreted via the urine.
- is clinically characterized by:
- Progressive mental deterioration usually pronounced by age 1.
- Seizures.
- Hyperactivity & other neurologic abnormalities.
- Decreased pigmentation of hair, eyes, & skin. (Children are characteristically blond & blue-eyed).
- Mousy body odour from phenylacetic acid in urine & sweat.
= can be successfully treated by a phenylalanine–free diet.
= Screening tests for serum phenylalanine or urinary catabolites are ordinarily performed on the 3rd or 4th day of life . But this is not currently done in Ethiopia.
3. X-linked recessive inheritance
All sex-linked disorders are X-linked. There is no Y-liked inheritance because Y-linked mutations result in infertility. X-linked disorders can be either recessive (almost all) or dominant (rare).
X-linked recessive inheritance:-
- is suspected when several male relatives in the female line of the family are affected.
a. Criteria:-
i. In the mating of a heterozygous carrier female parent & a normal male parent (the most frequent setting), the sons are hemizygous affected 50% of the time (i.e. the sons have 50% chance of being affected) & the daughters are normal heterozygous carriers 50% of the time & normal homozygotes 50% of the time. See Fig.4 below.
ii. Affected daughters are produced by matings of heterozygous females with affected males.
iii. No male-to-male (i.e. father-to-son) transmission of the trait (in all sex-linked inheritance). This is because a male contributes his Y chromosome to his son & does not contribute an X-chromosome to his son. On the other hand, since a male contributes his sole X-chromosome to each daughter, all daughters of a male with an X-linked disorder will inherit the mutant allele. All female offspring of affected males are carriers if the mother is normal.
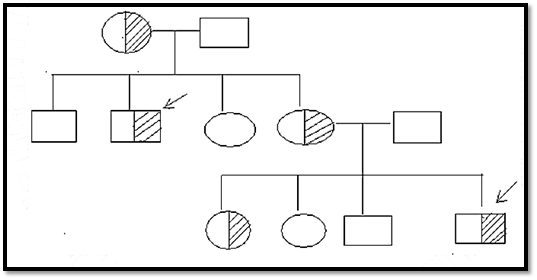
Fig.4. X-linked recessive pattern.
This figure shows an extended pedigree of an X-linked recessive disorder in which the male parents (in both generations) are normal & the female parents carriers. In contrast to the vertical distribution in dominant traits (parents & children affected) & the horizontal distribution in autosomal recessive traits (sibs affected), the pedigree pattern in X –linked recessive traits tends to be oblique, i.e. the trait manifests in the maternal uncles of affected males & in male cousins who are descended from the mother’s sisters who are carriers.
b. Pathogenesis of X-linked recessive disorders
The genes responsible for X-linked disorders are located on the X-chromosome, & the clinical risks are different for the 2 sexes.
Since a female has 2 X chromosomes, she may be either homozygous or heterozygous for a mutant gene, & the mutant allele may demonstrate either dominant or recessive expression. The homozygous female (i.e. having the mutation in both the X chromosomes) will express the full phenotypic change of the disease Clinical expression of X-linked recessive disorders in heterozygous females is often variable & is influenced by the normal random X-chromosome inactivation (i.e. lyonization) (See below). Normally, one of the two X-chromosomes in females is randomly inactivated. Therefore, in heterozygous females carrying X-linked recessive mutations, some cells have one active normal X chromosome & other cells have an active abnormal X chromosome containing the mutant allele. I.e. such females have a variable proportion of cells in which the mutant X-chromosome is active. Often the mutant allele is activated in only some of the cells. Therefore, the heterozygous female expresses the disorder partially & with less severity than hemizygous men. I.e. she usually does not express the full phenotypic change. E.g. G6PD deficiency. Very rarely, the mutant allele may be activated in most cells & this results in full expression of a heterozygous X-linked recessive condition in the female. In males, the Y chromosome is not homologous to the X-chromosome. So mutant genes on the X are not paired with alleles on the Y. The male is, therefore, said to be hemizygous (¬ heterozygous) for the X-linked mutant genes. Males have only oner X-chromosome, so they will clinically show the full phenotype of X-linked recessive diseases, regardless of whether the mutation produces a recessive or dominant allele in the female. Thus, the terms X-linked dominant or X-linked recessive refer only to the expression of the mutations in women.
c. Clinical examples of X-linked recessive disorders include:
- Hemophilia A & B
- Chronic granulomatous disease of childhood
- Glucose-6-phosphate dehydrogenase (G6PD) deficiency
- Agammaglobulinemia
- Wiskott -Aldrich syndrome
- Diabetes insipidus
- Lesch-Nyhan syndrome
- Fragile X syndrome
- Duchenne muscular dystrophy
X-linked dominant inheritance
- is a rare variant of X-linked inheritance.
- is when heterozygous females & hemizygous males phenotypically manifest the disorder.
- is caused by dominant disease alleles on the X-chromosome.
- is transmitted by an affected heterozygous female to half her sons & half her daughters.
- is transmitted by an affected male parent to all his daughters but none of his sons, if the female parent is unaffected.
- is exemplified by vitamin D-resistant rickets.
Mitochondrial inheritance
- is mediated by maternally transmitted mitochondrial genes, which are inherited exclusively by maternal transmission.
- is a rare form of inheritance mentioned here just for the sake of completeness.
References
Bezabeh ,M. ; Tesfaye,A.; Ergicho, B.; Erke, M.; Mengistu, S. and Bedane,A.; Desta, A.(2004). General Pathology. Jimma University, Gondar University Haramaya University, Dedub University.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|