


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-8-2016
Date: 21-3-2016
Date: 5-10-2020
|
Amorphous or Crystalline
Structurally, polymers in the solid state may be amorphous or crystalline. When polymers are cooled from the molten state or concentrated from the solution, molecules are often attracted to each other and tend to aggregate as closely as possible into a solid with the least possible potential energy. For some polymers, in the process of forming a solid, individual chains are folded and packed regularly in an orderly fashion. The resulting solid is a crystalline polymer with a long-range, three-dimensional, ordered arrangement. However, since the polymer chains are very long, it is impossible for the chains to fit into a perfect arrangement equivalent to that observed in low-molecular-weight materials. A measure of imperfection always exists. The degree of crystallinity, i.e., the fraction of the total polymer in the crystalline regions, may vary from a few percentage points to about 90% depending on the crystallization conditions. Examples of crystalline polymers include polyethylene (1), polyacrylonitrile (2), poly(ethylene terephthalate) (3), and polytetrafluoroethylene (4).
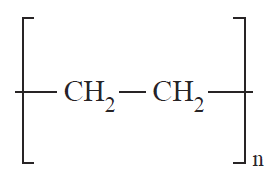
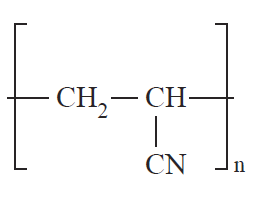
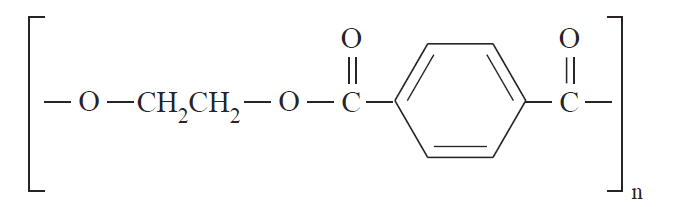
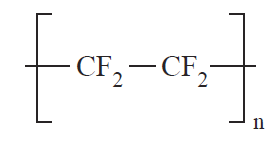
(1) (2) (3) (4)
In contrast to crystallizable polymers, amorphous polymers possess chains that are incapable of ordered arrangement. They are characterized in the solid state by a short-range order of repeating units. These polymers vitrify, forming an amorphous glassy solid in which the molecular chains are arranged at random and even entangled. Poly(methyl methacrylate) (23) and polycarbonate (30) are typical examples.
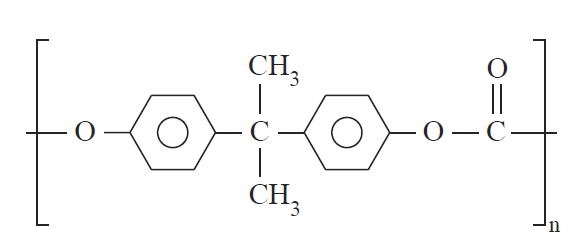
(5)
From the above discussion, it is obvious that the solid states of crystalline and amorphous polymers are characterized by a long-range order of molecular chains and a short-range order of repeating units, respectively. On the other hand, the melting of either polymer marks the onset of disorder. There are, however, some polymers which deviate from this general scheme in that the structure of the ordered regions is more or less disturbed. These are known as liquid crystalline polymers. They have phases characterized by structures intermediate between the ordered crystalline structure and the disordered fluid state. Solids of liquid crystalline polymers melt to form fluids in which much of the molecular order is retained within a certain range of temperature. The ordering is sufficient to impart some solidlike properties on the fluid, but the forces of attraction between molecules are not strong enough to
prevent flow. An example of a liquid crystalline polymer is polybenzamide (31).
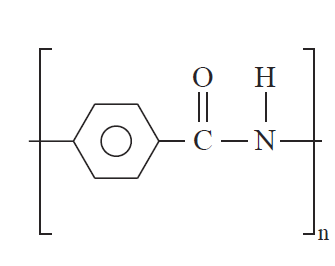
(6)
Liquid crystalline polymers are important in the fabrication of lightweight, ultra-high-strength, and temperature-resistant fibers and films such as Dupont’s Kevlar and Monsanto’s X-500. The structural factors responsible for promoting the above classes of polymers will be discussed when we treat the structure of polymers.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|