


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 21-4-2016
Date: 15-11-2020
Date: 15-4-2021
|
Diagonal Methods
It is often desirable to purify selectively those peptides from a protein that contain a particular amino acid, such as all the peptides containing cysteine residues. This is accomplished most elegantly by so-called “diagonal” techniques (1), which rely on a change in the properties of peptides caused by the selective modification of the amino acid residues of interest. If a mixture of peptides, generated by proteolysis of a protein for peptide mapping, is separated in one dimension and then subjected to the same procedure a second time, but at right angles to the first, all the peptides will lie on a diagonal because they had the same mobilities in both directions. The separation can be by electrophoresis or chromatography, but it should be carried out on a two-dimensional medium, such as paper or thin-layer plates. To detect certain peptides, and to cause them to have a different mobility in the second dimension, the entire mixture of peptides is treated after the first separation so as to modify all the residues of a particular type and to change their separation properties. After the second dimension separation, the peptides containing these residues consequently lie off the diagonal of all the other peptides and are readily identified and isolated.
1. Isolating Peptides Containing Certain Amino Acids
For example, peptides containing Cys residues can be purified by blocking their thiol groups by reaction with iodoacetic acid (Fig. 1a) (2). A two-dimensional diagonal peptide map is prepared by electrophoresis at pH 3.5, exposing the peptides to performic acid between the two separations. This oxidizes the sulfur atom of the modified Cys residues to the sulfone:
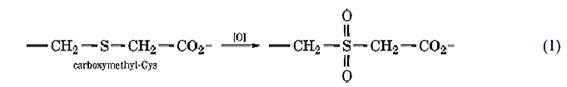
Which lowers the pKa value of the carboxyl group, so that peptides containing Cys residues are somewhat more acidic in the second dimension; they then lie to one side of the diagonal. Alternatively, the thiol groups can be reacted initially with N-ethylmaleimide; the N-ethylsuccinimide group introduced can then be hydrolyzed to the succinamic acid by treatment with ammonia after the first separation (3).

Figure 1. Isolation and identification of cysteine residues linked by disulfide bonds or modified by reaction with iodoacetate (2). The proteins were digested with trypsin, followed by chymotrypsin, and the resulting peptides were separated by paper electrophoresis at pH 3.5 in the horizontal direction (anode at the left). After exposure to performic acid vapor, the electrophoresis was repeated in the vertical direction (anode at bottom). The peptides were visualized by staining with ninhydrin. The diagonal indicated by the dashed line is defined by peptides that were not altered by the performic acid and consequently had the same mobility in both dimensions; many of these peptides have migrated off the paper. (a) Reduced BPTI (bovine pancreatic trypsin inhibitor) in which the six Cys residues have been blocked by reaction with iodoacetate. The six peptides containing these residues define a second diagonal, below the first, because they were slightly more acidic in the second dimension due to their oxidation to the sulfone. These peptides are identified by the numbers of the Cys residues they contain. The amino acid residues they contain are, starting from lower left to upper right, 54 to 58 (Cys55), 27 to 33 (Cys30), 5 to 15 (Cys5 and 14), 47 to 53 (Cys51), 1 to 15 (Cys5 and 14), and 36 to 39 (Cys38). (b) The major one-disulfide intermediate in the BPTI disulfide folding pathway that had been trapped by reacting all the four free Cys thiol groups with iodoacetate. The two peptides containing Cys30 and Cys51 are absent from the second diagonal of modified Cys residues and had the same mobility in the first dimension, indicating that they were originally linked by an intramolecular disulfide bond between these two Cys residues.
Analogous techniques have been developed for a few other residues, using appropriate chemical modifications specific for these residues. The amino groups of Lys-containing peptides are initially blocked with trifluroacetyl or maleyl groups, by treatment with the corresponding anhydride; these groups alter the charge and are easily removed after the first electrophoresis by acidification. Met residues are first alkylated with iodoacetamide to produce the charged sulfonium derivative. After the first electrophoresis, heating causes this derivative to cleave the polypeptide chain by a reaction analogous to that occurring in cleavage by cyanogen bromide. His residues are first blocked by dinitrophenylation of the side chain and then regenerated after the first electrophoresis by exposure of the peptides to a thiol-containing reagent. Arg residues are first blocked by reaction with cyclohexanedione and then regenerated at alkaline pH. Trp residues are modified by o-nitrophenylsulfenyl chloride after the first separation (4).
2. Characterizing Disulfide Bonds
Diagonal techniques were initially introduced by Brown and Hartley (1) for the determination of which pairs of Cys residues in a protein were linked by disulfide bonds. The protein is prepared for peptide mapping and the peptides are separated in the first dimension, under conditions where the disulfide bonds are stable and cannot rearrange or be reduced; the pairs of peptides linked by disulfide bonds consequently migrate together (Fig. 1b). The disulfide bonds are then cleaved by exposure to performic acid, which converts each such Cys residue to cysteic acid:

The peptides originally linked by the disulfide bond are now independent and more acidic. During separation in the second dimension, they migrate differently and lie off the diagonal, but they can be related by their common mobility in the second dimension.
Diagonal techniques are best suited to two-dimensional separations, such as paper electrophoresis and chromatography, which were used extensively in the past but have now been supplanted by more modern techniques that require less material, but operate in a single dimension, such as HPLC. The same basic technique can be used, but the fractions from the first separation must be separated again individually.
References
1. J. R. Brown and B. S. Hartley (1966) Biochem. J. 101, 214–228.
2. T. E. Creighton (1974) J. Mol. Biol. 87, 603–624.
3. H. Gehring and P. Christen (1980) Anal. Biochem. 107, 35–361.
4. T. Sasagawa et al. (1983) Anal. Biochem. 134, 224–229.



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
مشاتل الكفيل تزيّن مجمّع أبي الفضل العبّاس (عليه السلام) بالورد استعدادًا لحفل التخرج المركزي
|
|
|