


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 2023-07-01
التاريخ: 26-2-2016
التاريخ: 2023-08-29
التاريخ: 2023-07-31
|
The E2 reaction occurs most easily if the molecule undergoing reaction can assume a conformation, 2, in which the leaving groups, H and X, are trans to each other and the atoms H−Cβ−Cα−X lie in one plane. Elimination then proceeds from opposite sides of the incipient double bond to give an alkene of structure 3. We shall call this mode of elimination antarafacial to distinguish
Figure 8-6: Schematic representation of antarafacial (literally "opposite-face") and suprafacial (literally "above-face") elimination or addition of a reagent X−Y to ethene
it from another possible mode of elimination that is called suprafacial. (See Figure 8-6).
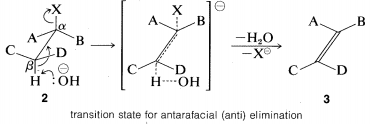
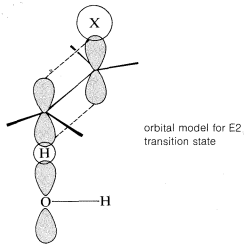
The transition state for conversion of 2 to 3 is particularly reasonable because it combines some of the geometry of both the reactants and the products and therefore gives the best overlap of the reacting orbitals necessary for the formation of the π bond. This is shown more explicitly below.
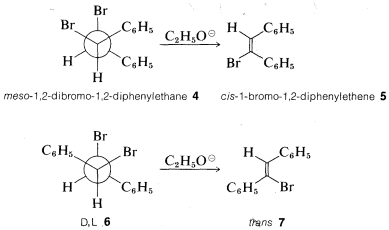
As an illustration of the stereospecificity of eliminations, the meso compound 4 gives the cis-alkene 5, whereas the D,L isomers 6 give the trans-alkene 7 with ethoxide. Both reactions clearly proceed by antarafacial elimination:
When antarafacial elimination is rendered difficult by the inability of the reacting groups to acquire the desired trans arrangement, then suprafacial elimination can occur, although less readily. An example is chlorocyclopentane, in which H and X cannot assume a trans configuration without very considerable strain but which does undergo suprafacial elimination at a reasonable rate:
Figure 8-6. At the risk of annoying those who often rightly dislike complicated names for simple processes, we have chosen to adopt the proposal of R. B. Woodward and R. Hoffman, that elimination (or addition) which involves a same-side cleavage (or formation) of bonds be called suprafacial and the opposite-side cleavage (or formation) of bonds be called antarafacial. The alternative of using syn for suprafacial and anti for antarafacial would be simpler and easier to remember, but the terms syn and anti already are used for configurations, which is exactly what we want to avoid. The problem with the terms cis elimination (or addition) and trans elimination (or addition) is that they do not necessarily lead to products that, by other conventions, are understood to be cis and trans products, respectively, For example, to say that "trans elimination leads to cis product" is a needless confusion. It is much clearer to say that "antarafacial elimination gives the cis product" - and now there is no confusion of the mode of elimination with whatever stereochemical convention identifies the product.
Persuasive arguments have been made that many E2 reactions proceed by the sequence
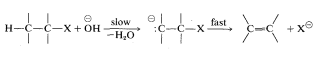
If this is so, antarafacial elimination still is predicted to be favored.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|