


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 19-10-2020
التاريخ: 2024-04-24
التاريخ: 8-9-2020
التاريخ: 31-10-2016
|
Of the two mechanisms, A requires that the reaction rate be determined solely by the rate of the first step . This means that the rate at which methanol is formed (measured in moles per unit volume per unit time) will depend on the chloromethane concentration, but not on the hydroxide ion concentration, because hydroxide ion is not utilized except in a fast secondary reaction. In contrast, Mechanism BB requires the rate to depend on the concentrations of both reagents because the slow step involves collisions between hydroxide ions and chloromethane molecules.
v=kA[CH3Cl](8.5.1)(8.5.1)
v=kB[CH3Cl][OH−](8.5.2)
More precisely, the reaction rate (ν) may be expressed in terms of Equation 8.5.1 for Mechanism A and Equation 8.5.2 for Mechanism B:
Customarily, ν is expressed in moles of product formed per liter of solution per unit of time (most frequently in seconds). The concentration terms [CH3Cl] and [OH⊖] are then in units of moles per liter, and the proportionality constant kk (called the specific rate constant) has the units of sec−1 for Mechanism A and mol−1×L×sec−1 for Mechanism B.
It is important to recognize the difference between the order of a reaction with respect to a specific reactant and the overall order of a reaction. The order of a reaction with respect to a particular reactant is the power to which the concentration of that reactant must be raised to have direct proportionality between concentration and reaction rate. According to Equation 8.5.2 the rate of the chloromethane-hydroxide ion reaction is first order with respect to chloromethane and first order with respect to hydroxide ion. In Equation 8.5.1 the rate is first order with respect to chloromethane and zero order with respect to hydroxide ion because [OH⊖]0=1. The overall order of reaction is the sum of the orders of the respective reactants. Thus Equations 8.5.1 and 8.5.2 express the rates of overall first-order and second-order reactions, respectively.
We can use the overall reaction order to distinguish between the two possible mechanisms, A and B. Experimentally, the rate of formation of methanol is found to be proportional to the concentrations both of chloromethane and of hydroxide ion. Therefore the reaction rate is second order overall and is expressed correctly by Equation 8.5.2. This means that the mechanism of the reaction is the single-step process B. Such reactions generally are classified as bimolecular nucleophilic substitutions, often designated SN2, S for substitution, N for nucleophilic, and 2 for bimolecular, because there are two reactant molecules in the transition state. To summarize: For an SN2 reaction,
v=k[RX][Y](8.5.3)
mechanism:
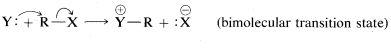
The stepwise Mechanism AA is a unimolecular nucleophilic substitution and accordingly is designated SN1SN1. The numeral 1 (or 2) used in these designations does not refer to the kinetic order of the reaction, but refers to the number of molecules (not including solvent molecules) that make up the transition state. Thus for SN1,

or
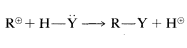



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|