


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 31-12-2021
Date: 1-11-2021
Date: 17-9-2021
|
Cholesterol Synthesis
Cholesterol is synthesized by virtually all tissues in humans, although liver, intestine, adrenal cortex, and reproductive tissues, including ovaries, testes, and placenta, make the largest contributions to the cholesterol pool. As with FA, all the carbon atoms in cholesterol are provided by acetyl coenzyme A (CoA), and nicotinamide adenine dinucleotide phosphate (NADPH) provides the reducing equivalents. The pathway is endergonic, being driven by hydrolysis of the highenergy thioester bond of acetyl CoA and the terminal phosphate bond of ATP.
Synthesis requires enzymes in the cytosol, the membrane of the smooth endoplasmic reticulum (SER), and the peroxisome. The pathway is responsive to changes in cholesterol concentration, and regulatory mechanisms exist to balance the rate of cholesterol synthesis against the rate of cholesterol excretion.
An imbalance in this regulation can lead to an elevation in circulating levels of plasma cholesterol, with the potential for vascular disease.
A. 3-Hydroxy-3-methylglutaryl coenzyme A synthesis
The first two reactions in the cholesterol biosynthetic pathway are similar to those in the pathway that produces ketone bodies . They result in the production of 3-hydroxy-3-methylglutaryl CoA ([HMG CoA], Fig. 1). First, two acetyl CoA molecules condense to form acetoacetyl CoA. Next, a third molecule of acetyl CoA is added by HMG CoA synthase, producing HMG CoA, a six-carbon compound. [Note: Liver parenchymal cells contain two isoenzymes of the synthase. The cytosolic enzyme participates in cholesterol synthesis, whereas the mitochondrial enzyme functions in the pathway for ketone body synthesis.]

Figure 1: Synthesis of HMG CoA. CoA = coenzyme A.
B. Mevalonate synthesis
HMG CoA is reduced to mevalonate by HMG CoA reductase. This is the rate-limiting and key regulated step in cholesterol synthesis. It occurs in the cytosol, uses two molecules of NADPH as the reducing agent, and releases CoA, making the reaction irreversible (Fig. 2). [Note: HMG CoA reductase is an integral membrane protein of the SER, with its catalytic domain projecting into the cytosol.

Figure 2: Synthesis of mevalonate. HMG CoA = hydroxymethylglutaryl coenzyme A; NADP(H) = nicotinamide adenine dinucleotide phosphate.
C. Cholesterol synthesis from mevalonate
The reactions and enzymes involved in the synthesis of cholesterol from mevalonate are illustrated in Figure 3. [Note: The numbers shown in brackets below correspond to numbered reactions shown in this figure.]
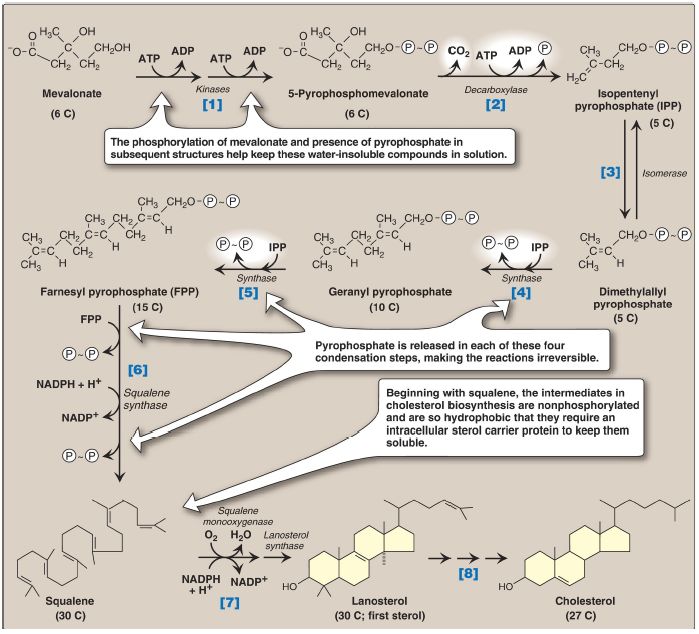
Figure 3: Synthesis of cholesterol from mevalonate. ADP = adenosine diphosphate; = phosphate; ∼ = pyrophosphate; NADP(H) = nicotinamide adenine dinucleotide phosphate.
[1] Mevalonate is converted to 5-pyrophosphomevalonate in two steps, each of which transfers a phosphate group from ATP.
[2] A five-carbon isoprene unit, isopentenyl pyrophosphate (IPP), is formed by the decarboxylation of 5-pyrophosphomevalonate. The reaction requires ATP. [Note: IPP is the precursor of a family of molecules with diverse functions, the isoprenoids. Cholesterol is a sterol isoprenoid. Nonsterol isoprenoids include dolichol and ubiquinone (or, coenzyme .]
[3] IPP is isomerized to 3,3-dimethylallyl pyrophosphate (DPP).
[4] IPP and DPP condense to form 10-carbon geranyl pyrophosphate (GPP).
[5] A second molecule of IPP then condenses with GPP to form 15-carbon farnesyl pyrophosphate (FPP). [Note: Covalent attachment of farnesyl to proteins, a process known as prenylation, is one mechanism for anchoring proteins (for example, ras) to the inner face of plasma membranes.]
[6] Two molecules of FPP combine, releasing pyrophosphate, and are reduced, forming the 30-carbon compound squalene. [Note: Squalene is formed from six isoprenoid units. Because 3 ATP are hydrolyzed per mevalonate residue converted to IPP, a total of 18 ATP are required to make the polyisoprenoid squalene.]
[7] Squalene is converted to the sterol lanosterol by a sequence of two reactions catalyzed by SER-associated enzymes that use molecular oxygen (O2) and NADPH. The hydroxylation of linear squalene triggers the cyclization of the structure to lanosterol.
[8] The conversion of lanosterol to cholesterol is a multistep process involving shortening of the side chain, oxidative removal of methyl groups, reduction of double bonds, and migration of a double bond. Smith-Lemli-Opitz syndrome (SLOS), an autosomal-recessive disorder of cholesterol biosynthesis, is caused by a partial deficiency in 7-dehydrocholesterol-7-reductase, the enzyme that reduces the double bond in 7-dehydrocholesterol (7-DHC), thereby converting it to cholesterol. SLOS is one of several multisystem, embryonic malformation syndromes associated with impaired cholesterol synthesis. [Note: 7-DHC is converted to vitamin D3 in the skin ).]



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|