


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية| The Eukaryotic Growth Factor Signal Transduction Pathway Promotes Entry to S Phase |
|
|
|
Read More
Date: 15-5-2016
Date: 20-12-2015
Date: 27-12-2015
|
The Eukaryotic Growth Factor Signal Transduction Pathway Promotes Entry to S Phase
KEY CONCEPTS
- The function of a growth factor is to stabilize dimerization of its receptor and subsequent phosphorylation of the cytoplasmic domain of the receptor.
- The function of the growth factor receptor is to recruit the exchange factor SOS to the membrane to activate RAS.
- The function of activated RAS is to recruit RAF to the membrane to become activated.
- The function of RAF is to initiate a phosphorylation cascade leading to the phosphorylation of a set of transcription factors that can enter the nucleus and begin S phase.
The vast majority of eukaryotic cells in a multicellular individual are not growing; that is, they are in the cell cycle stage of G0, as we saw in the beginning of this chapter. Stem cells and most embryonic cells, however, are actively growing. A growing cell exiting mitosis has two choices—it can enter G1 and begin a new round of cell division or it can stop dividing and enter G0, a quiescent stage and, if so programmed, begin differentiation. This decision is controlled by the developmental history of the cell and the presence or absence of growth factors and their receptors.
For a cell to begin the cell cycle from G0, or continue to divide after M phase, it must be programmed to express the proper growth factor receptor gene. Elsewhere in the organism, typically in a master gland (but can also occur in neighboring cells), the gene for the proper growth factor must be expressed. The signal transduction pathway is the biochemical mechanism by which the growth factor signal to grow is communicated from its source outside of the cell into the nucleus to ultimately cause that cell to begin replication and growth. The pathway that we describe in this section is universal in eukaryotes, ranging from yeast to humans.
The genes that encode elements of the signal transduction pathway are proto-oncogenes, genes that when altered can cause cancer. As an example of this pathway, we examine Epidermal Growth Factor (EGF) and its receptor, EGFR—a member of the erbB family of four related receptors. These two proteins, EGF and EGFR, and the genes that encode them are the first two elements in the pathway. EGF is a peptide hormone (as opposed to a steroid hormone such as estrogen). The EGFR specifically binds EGF in a lock-and-key type of mechanism. EGFR is a one-pass membrane protein in the family known as receptor tyrosine kinases (RTK), as shown in FIGURE 1a. The receptor has an external domain (that is outside the cell) that binds EGF, a single membrane-spanning domain, and an internal cytoplasmic domain with intrinsic tyrosine kinase activity. The local membrane composition (e.g., cholesterol) can modulate the dynamics of the signal transduction pathway.
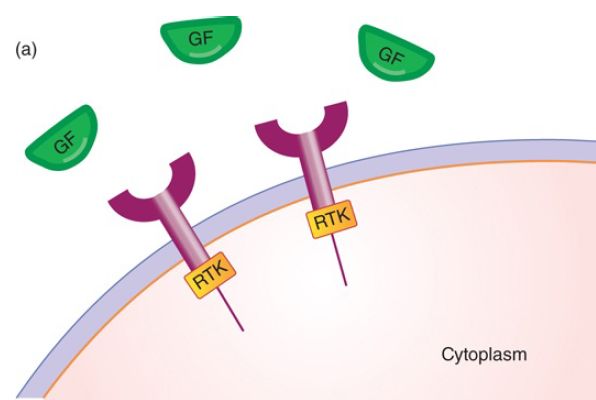

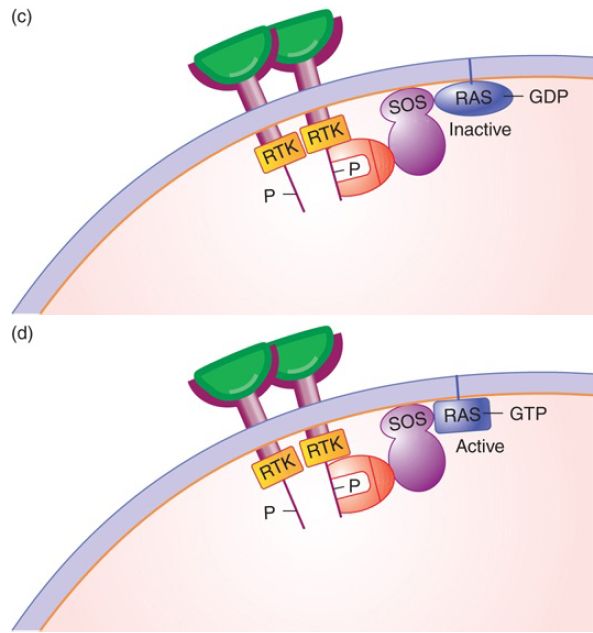
FIGURE 1. The signal transduction pathway. (a) Growth factors and growth factor receptors: The growth factor extracellular domain will bind the growth factor in a lock-and-key fashion. The growth factor receptor intracellular domain contains an intrinsic protein kinase domain called RTK. (b) Growth factor binding to its receptor will stabilize receptor dimerization, leading to phosphorylation of each cytoplasmic domain on tyrosine. The phosphotyrosine residues can serve as binding sites for proteins such as Grb2, shown here. (c) Grb2 binds the Tyr-P so that its binding partner SOS, a guanosine nucleotide exchange factor, is brought to the membrane and can activate the inactive RAS-GDP. (d) SOS removes the GDP, replacing it with GTP, activating RAS.
Hormone binding to receptor stabilizes receptor dimerization (usually homodimerization, but heterodimers with other erbB family members can occur), which leads to multiple cross-phosphorylation events of each receptor’s cytoplasmic domain. The only function of the hormone is to stabilize receptor dimerization. Each receptor phosphorylates the other on a set of five tyrosine amino acid residues in the cytoplasmic domain, as shown in FIGURE 1b.
Each phosphorylated tyrosine (Tyr-P) serves as a docking site for a specific adaptor protein to bind to the receptor, as shown in FIGURE 1c. We will examine a single pathway, but it is important to keep in mind that cells contain many different receptors that are active at the same time, and each receptor has multiple docking sites for multiple proteins. The reality is that it is not a pathway but rather an information network.
Paradoxically, hormone binding to the receptor also causes clathrin-mediated endocytosis of the hormone receptor complex to the lysosomal complex, where it is targeted for destruction, and thus turnover. This trafficking is regulated by microtubule deacetylation, which controls the proportion of receptors that are returned to the surface. This is part of an important attenuation mechanism to prevent accidental triggering of the pathway and it means that growth factor must be continually present to propagate a sustained signal.
The third member of the signal transduction pathway is the RAS protein (encoded by the ras gene). RAS is a member of a large family of G-proteins, proteins that bind a guanosine nucleotide, either GTP (for the active form of RAS) or GDP (for the inactive form). RAS is connected to the membrane by a prenylated (lipid) tail, and typically found in nanoclusters on the cytoplasmic side of the membrane to enhance downstream signaling. To continue the flow of information through the signal transduction pathway communicating that a growth factor is present, inactive RAS must be converted from RAS-GDP to RAS-GTP by a protein called Son
of Sevenless (SOS), a guanosine nucleotide exchange factor (GEF) that exchanges GTP for GDP. Its function is to remove the GDP from RAS and replace it with GTP, as shown in FIGURE 1d. RAS also has a weak intrinsic phosphatase (GTPase) activity that slowly converts GTP to GDP. Again, this provides a mechanism to ensure that growth factor must be present continually for the signal to propagate.
To activate RAS, SOS must be specifically recruited to the membrane in order to interact with RAS-GDP. It is the membrane phospholipids themselves that serve to unlock an auto-inhibitory domain so that SOS can bind to RAS. SOS is in a complex with an adaptor protein called Grb2, an interesting protein with two domains: an SH2 domain that binds Tyr-P, and an SH3 domain that binds proteins containing another SH3 domain. The specificity for binding to the receptor lies in the amino acids surrounding each Tyr-P. The only function of the growth factor is to stabilize dimerization of the receptor, which leads to its phosphorylation, which in turn leads to recruitment of SOS to the membrane to activate RAS.
Inactive RAS-GDP and active RAS-GTP are in a dynamic equilibrium controlled by the exchange factor GEF and another set of proteins that stimulate the intrinsic GTPase of RAS, such as RAS GAP (GTPase activating protein).
ras oncogenic mutations that constitutively activate RAS are among the most frequent oncogenic mutations found in tumors. The most common mutation is a single nucleotide change that causes a single amino acid change, resulting in altered function. RAS has a key altered property: It binds GTP with a higher affinity than GDP. The consequence is that it no longer requires a growth factor to trigger activation; it is constitutively active. This kind of mutation is referred to as a dominant gain-of-function mutation.
Activated RAS, RAS-GTP, now itself serves as a docking site to recruit the fourth member of the pathway to be activated: a structurally inactive form of RAF (also known as MAPKKK or
mitogen-activated protein kinase kinase kinase), a serine/threonine protein kinase. The activation of RAF on the membrane has been one of the most baffling steps, with researchers having proposed many models over the years. The only function of RAS-GTP is to recruit RAF to the membrane for activation; it does nothing else.
The most recent model is the dimer model for RAS-mediated activation of a dimer of RAF (see Figure 2). This activation is facilitated by the fact that RAS is present in the membrane in high concentration in nanoclusters. This high concentration of RAS leads to the formation of a dimer of RAS-GTP which facilitates the next step. RAF activation on the membrane involves its dimerization leading to the RAS-assisted unfolding of the autoinhibitory domains of the RAF dimer. This then allows phosphorylation by another ONC membrane associated kinase, SRC, and release of the RAF dimer from the platform.
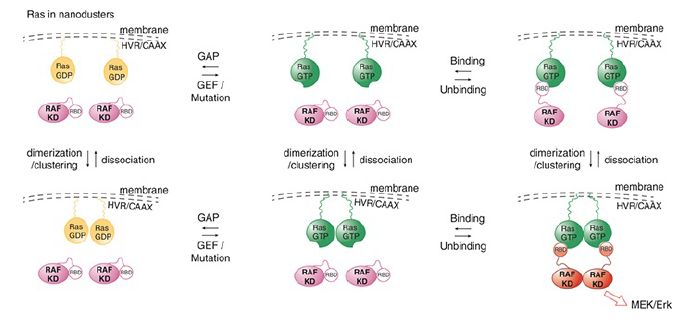
FIGURE 2. Dimer model for Ras-mediated activation of Raf. Ras-GTP forms dimers to cooperatively activate Raf.
Activated RAF phosphorylates a second kinase, such as one of the mitogen-activated kinase (MEK) factors, which then phosphorylates a third kinase, such as one of the extracellular signal-regulated kinase (ERK) factors, which can then phosphorylate and activate the set of transcription factors such as MYC, JUN, and FOS. This allows their entry into the nucleus to begin transcribing the genes to prepare for transit through G1 and entry into S phase. Again, note that this is a description of a single pathway within a network that has extensive crosstalk between members. In addition, this kinase cascade is modulated by an extensive network of phosphatases.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|