
 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 2-8-2020
Date: 10-8-2020
Date: 11-6-2019
|
We can apply our knowledge of quantum numbers to describe the arrangement of electrons for a given atom. We do this with something called electron configurations. They are effectively a map of the electrons for a given atom. We look at the four quantum numbers for a given electron and then assign that electron to a specific orbitals below.
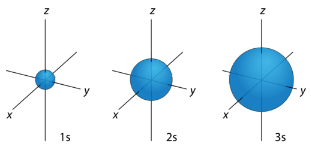
Figure 1: s orbitals have no orientational preference and resemble spheres.
orbitals has a different orientation in three-dimensional space.
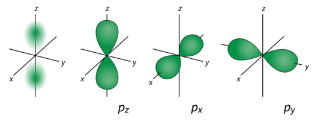
Figure 2 : p orbitals have an orientational preference and resemble dumbells. d Orbitals When l=2 , ml values can be −2,−1,0,+1,+2 for a total of five d orbitals. Note that all five of the orbitals have specific three-dimensional orientations.
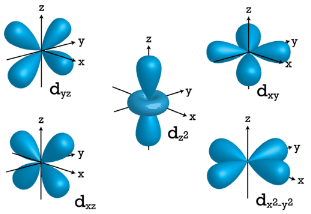
Figure 3 :d orbitals have an orientational preference and exhibit complex structures. f Orbitals The most complex set of orbitals are the f orbitals. When l=3, ml values can be −3,−2,−1,0,+1,+2,+3 for a total of seven different orbital shapes. Again, note the specific orientations of the different f
orbitals.
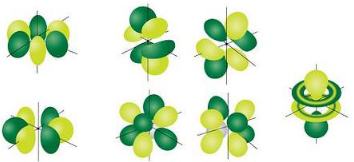
Figure 4 : f orbitals have an orientational preference and exhibit quite complex structures.
Orbitals that have the same value of the principal quantum number form a shell. Orbitals within a shell are divided into subshells that have the same value of the angular quantum number. Some of the allowed combinations of quantum numbers are compared in Table 1:
Table 1: Electron Arrangement Within Energy Levels



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|