


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-10-2020
Date: 11-9-2019
Date: 5-8-2018
|
It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. The dominant factor here is hydrogen bonding, and the first table below documents the powerful intermolecular attraction that results from -O-H---O- hydrogen bonding in alcohols (light blue columns). Corresponding -N-H---N- hydrogen bonding is weaker, as the lower boiling points of similarly sized amines (light green columns) demonstrate. Alkanes provide reference compounds in which hydrogen bonding is not possible, and the increase in boiling point for equivalent 1º-amines is roughly half the increase observed for equivalent alcohols.
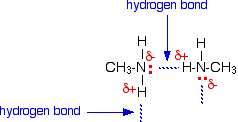
| Compound | CH3CH3 | CH3OH | CH3NH2 | CH3CH2CH3 | CH3CH2OH | CH3CH2NH2 |
|---|---|---|---|---|---|---|
| Mol.Wt. | 30 | 32 | 31 | 44 | 46 | 45 |
| Boiling Point ºC |
-88.6º | 65º | -6.0º | -42º | 78.5º | 16.6º |
The second table illustrates differences associated with isomeric 1º, 2º & 3º-amines, as well as the influence of chain branching. Since 1º-amines have two hydrogens available for hydrogen bonding, we expect them to have higher boiling points than isomeric 2º-amines, which in turn should boil higher than isomeric 3º-amines (no hydrogen bonding). Indeed, 3º-amines have boiling points similar to equivalent sized ethers; and in all but the smallest compounds, corresponding ethers, 3º-amines and alkanes have similar boiling points. In the examples shown here, it is further demonstrated that chain branching reduces boiling points by 10 to 15 ºC.
| Compound | CH3(CH2)2CH3 | CH3(CH2)2OH | CH3(CH2)2NH2 | CH3CH2NHCH3 | (CH3)3CH | (CH3)2CHOH | (CH3)2CHNH2 | (CH3)3N |
|---|---|---|---|---|---|---|---|---|
| Mol.Wt. | 58 | 60 | 59 | 59 | 58 | 60 | 59 | 59 |
| Boiling Point ºC |
-0.5º | 97º | 48º | 37º | -12º | 82º | 34º | 3º |
The water solubility of 1º and 2º-amines is similar to that of comparable alcohols. As expected, the water solubility of 3º-amines and ethers is also similar. These comparisons, however, are valid only for pure compounds in neutral water. The basicity of amines (next section) allows them to be dissolved in dilute mineral acid solutions, and this property facilitates their separation from neutral compounds such as alcohols and hydrocarbons by partitioning between the phases of non-miscible solvents.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|