


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-7-2019
Date: 10-7-2018
Date: 15-1-2020
|
The most common example of sulfonium ions in a living organism is the reaction of S-Adenosylmethionine. Some of the most important examples of SN2 reactions in biochemistry are those catalyzed by S-adenosyl methionine (SAM) – dependent methyltransferase enzymes. We have already seen, in chapter 6 and again in chapter 8, how a methyl group is transferred in an SN2 reaction from SAM to the amine group on the nucleotide base adenosine:

(Nucleic Acids Res. 2000, 28, 3950).
Another SAM-dependent methylation reaction is catalyzed by an enzyme called catechol-O-methyltransferase. The substrate here is epinephrine, also known as adrenaline.
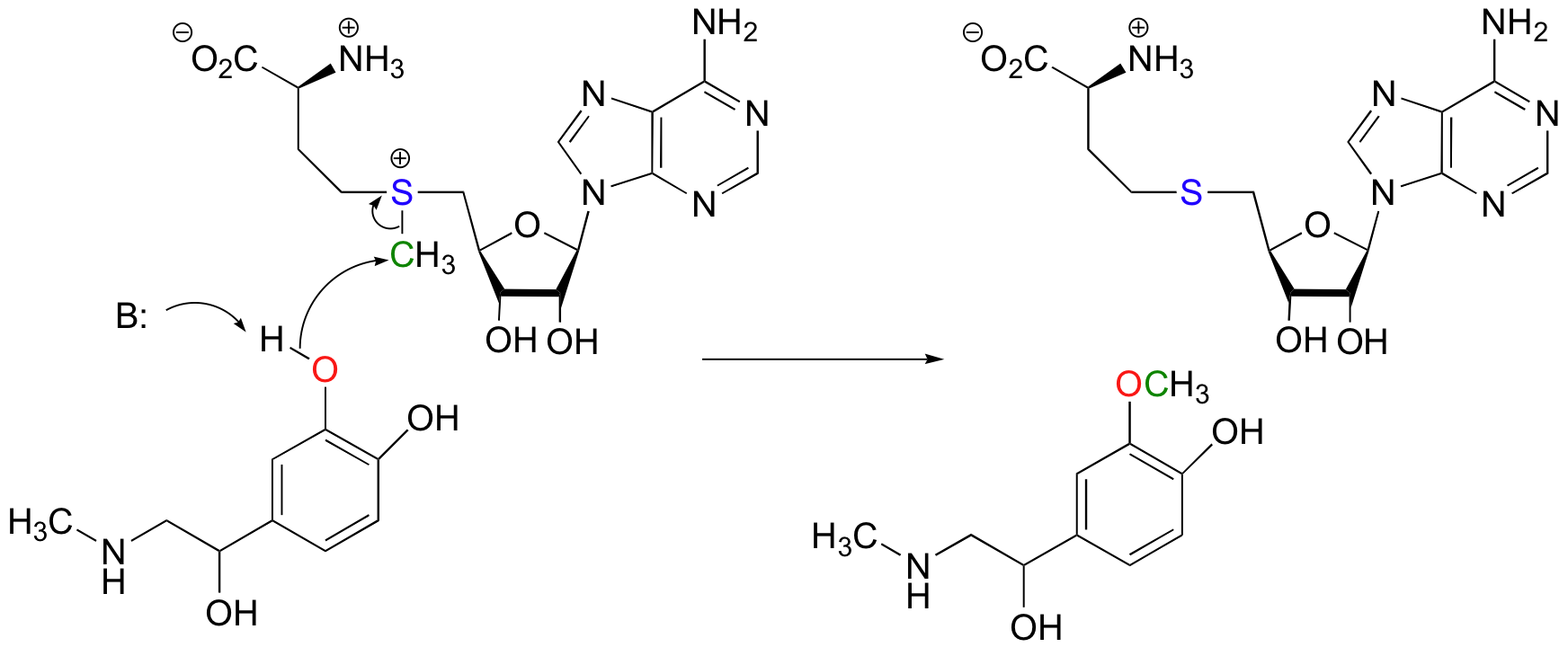
Notice that in this example, the attacking nucleophile is an alcohol rather than an amine (that’s why the enzyme is called an O-methyltransferase). In both cases, though, a basic amino acid side chain is positioned in the active site in just the right place to deprotonate the nucleophilic group as it attacks, increasing its nucleophilicity. The electrophile in both reactions is a methyl carbon, so there is little steric hindrance to slow down the nucleophilic attack. The methyl carbon is electrophilic because it is bonded to a positively-charged sulfur, which is a powerful electron withdrawing group. The positive charge on the sulfur also makes it an excellent leaving group, as the resulting product will be a neutral and very stable sulfide. All in all, in both reactions we have a reasonably good nucleophile, an electron-poor, unhindered electrophile, and an excellent leaving group.
Because the electrophilic carbon in these reactions is a methyl carbon, a stepwise SN1-like mechanism is extremely unlikely: a methyl carbocation is very high in energy and thus is not a reasonable intermediate to propose. We can confidently predict that this reaction is SN2. Does this SN2 reaction occur, as expected, with inversion of stereochemistry? Of course, the electrophilic methyl carbon in these reactions is achiral, so inversion is not apparent. To demonstrate inversion, the following experiment has been carried out with catechol-O-methyltransferase:
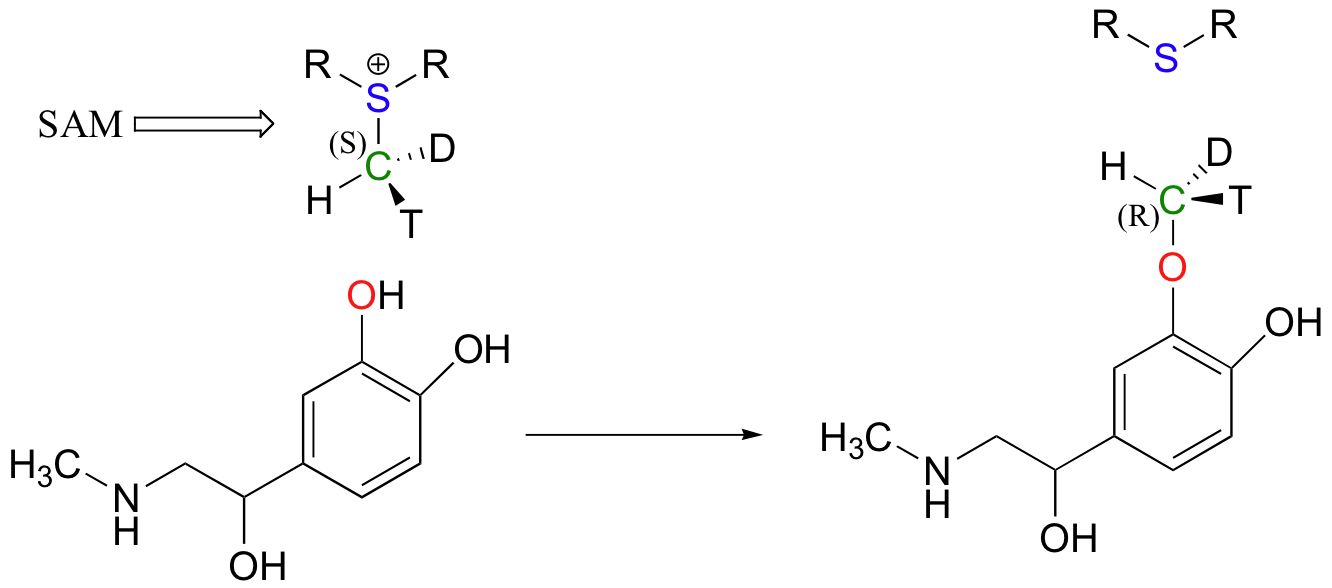
Here, the methyl group of SAM was made to be chiral by incorporating hydrogen isotopes tritium (3H, T) and deuterium (2H, D). The researchers determined that the reaction occurred with inversion of configuration, as expected for an SN2 displacement (J. Biol. Chem. 1980, 255, 9124).
Sulfides can be easily oxidized. Reacting a sulfide with hydrogen peroxide, H2O2, as room termpeature produces a sulfoxide (R2SO). The oxidation can be continued by reaction with a peroxyacid to produce the sulfone (R2SO2)
A common example of a sulfoxide is the solvent dimethyl sulfoxide (DMSO). DMSO is polar aprotic solvent.
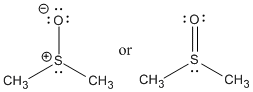
Figure 1.1. DMSO is a very polar, aprotic solvent.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|