


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 29-9-2020
Date: 1-8-2019
Date: 10-7-2019
|
Variation of rates when you change the alkene
This applies to unsymmetrical alkenes as well as to symmetrical ones. For simplicity the examples given below are all symmetrical ones- but they don't have to be. Reaction rates increase as the alkene gets more complicated - in the sense of the number of alkyl groups (such as methyl groups) attached to the carbon atoms at either end of the double bond.
For example:
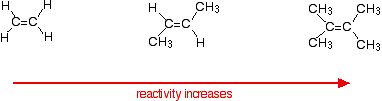
There are two ways of looking at the reasons for this - both of which need you to know about the mechanism for the reactions.
Alkenes react because the electrons in the pi bond attract things with any degree of positive charge. Anything which increases the electron density around the double bond will help this. Alkyl groups have a tendency to "push" electrons away from themselves towards the double bond. The more alkyl groups you have, the more negative the area around the double bonds becomes.
The more negatively charged that region becomes, the more it will attract molecules like hydrogen chloride. The more important reason, though, lies in the stability of the intermediate ion formed during the reaction. The three examples given above produce these carbocations (carbonium ions) at the half-way stage of the reaction:
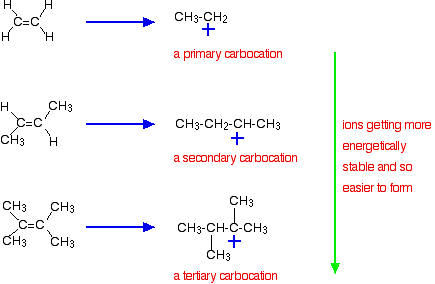
The stability of the intermediate ions governs the activation energy for the reaction. As you go towards the more complicated alkenes, the activation energy for the reaction falls. That means that the reactions become faster.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|