

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Preparation of Ylides
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
7-9-2018
1660
Preparation of Ylides
It has been noted that dipolar phosphorus and sulfur oxides are stabilized by p-d bonding. This may be illustrated by a resonance description, as shown here.
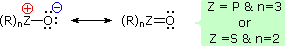
This bonding stabilization extends to carbanions alpha to phosphonium and sulfonium centers, and the zwitterionic conjugate bases derived from such cations are known as ylides. Approximate pKa's for some ylide precursors and related compounds are provided in the following table. The acidic hydrogen atoms are colored red. By convention, pKa's are usually adjusted to conform to the standard solvent water; however, in practice, measurements of very weak acids are necessarily made in non-aqueous solvents such as DMSO (dimethyl sufoxide). The green numbers in the table represent DMSO measurements, and although these are larger than the aqueous approximations, the relative order is unchanged. Note that DMSO itself is the weakest acid of those shown.
| Compound | (C6H5)3P(+)–CH3 I(–) | (CH3)3S(+) I(–) | (CH3)3S(+)=O I(–) | (CH3)2S=O | (CH3)2S(=O)2 | CH3-P(OCH3)2=O |
|---|---|---|---|---|---|---|
| Name | methyltriphenylphosphonium iodide |
trimethylsulfonium iodide |
trimethylsulfoxonium iodide |
dimethyl sulfoxide | dimethyl sulfone | dimethyl methylphosphonate |
| pKa DMSO |
17 22 |
20 25 |
15 18 |
30 35 |
26 31 |
25 31 |
Some characteristic preparations of ylide reagents are shown below. Very strong bases, such as butyl lithium, are required for complete formation of ylides. Sodium hydride (NaH), another powerful base, is insoluble in most solvents, but its reaction with DMSO (the weakest acid in the table) generates a strong conjugate base, CH3)S(=O)CH2(–) Na(+), known as dimsyl sodium. This soluble base is widely used for the generation of ylides in DMSO solution.
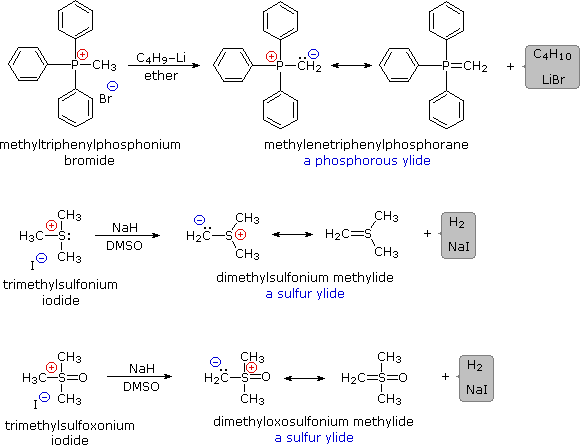
The ylides shown here are all strong bases. Like other strongly basic organic reagents, they are protonated by water and alcohols, and are sensitive to oxygen. Water decomposes alkylidenephosphoranes to hydrocarbons and phosphine oxides, as shown. Oxygen cleaves these ylides in a similar fashion, the alkylidene moiety being converted to a carbonyl compound.
R3P=CR'2 + H2O  [ R3P(OH)–CHR'2 ] [ R3P(OH)–CHR'2 ]  R3P=O + R'2CH2 R3P=O + R'2CH2 |
R3P=CR'2 + O2  R3P=O + R'2C=O R3P=O + R'2C=O |
 الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
الاكثر قراءة في تجارب وتفاعلات في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















