
ADDITION POLYMERIZATION
 المؤلف:
sami matar & Lewis. F. Hatch
المؤلف:
sami matar & Lewis. F. Hatch
 المصدر:
Chemistry of PETROCHEMICAL PROCESSES
المصدر:
Chemistry of PETROCHEMICAL PROCESSES
 الجزء والصفحة:
p 304
الجزء والصفحة:
p 304
 18-9-2017
18-9-2017
 1859
1859
ADDITION POLYMERIZATION
Addition polymerization is employed primarily with substituted or unsubstituted olefins and conjugated diolefins. Addition polymerization initiators are free radicals, anions, cations, and coordination compounds.
In addition polymerization, a chain grows simply by adding monomer molecules to a propagating chain. The first step is to add a free radical, a cationic or an anionic initiator (IZ) to the monomer. For example, in ethylene polymerization (with a special catalyst), the chain grows by attaching the ethylene units one after another until the polymer terminates. This type of addition produces a linear polymer:

Branching occurs especially when free radical initiators are used due to chain transfer reactions (see following section, “Free Radical Polymerizations”). For a substituted olefin (such as vinyl chloride), the addition primarily produces the most stable intermediate (I). Intermediate (II) does not form to any appreciable extent:
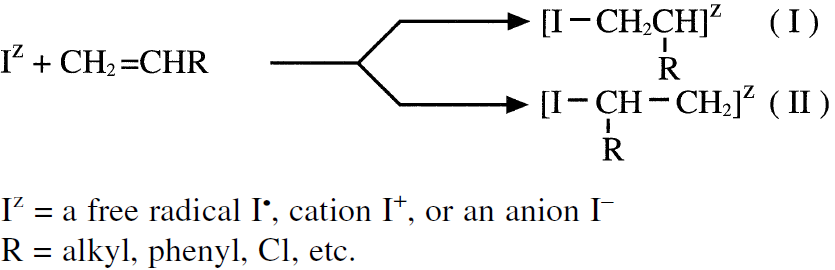
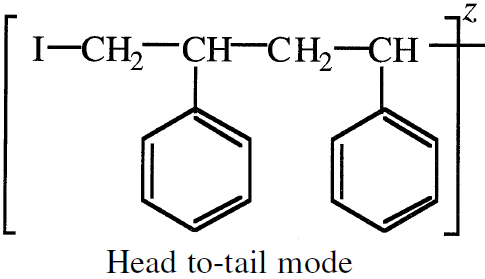
Propagation then occurs by successive monomer molecules additions to the intermediates. Three addition modes are possible: (a) Head to tail; (b) Head to head, and (c) tail to tail. The head-to-tail addition mode produces the most stable intermediate. For example, styrene polymerization mainly produces the head-totail intermediate:
Head-to-head or tail-to-tail modes of addition are less likely because the intermediates are generally unstable:
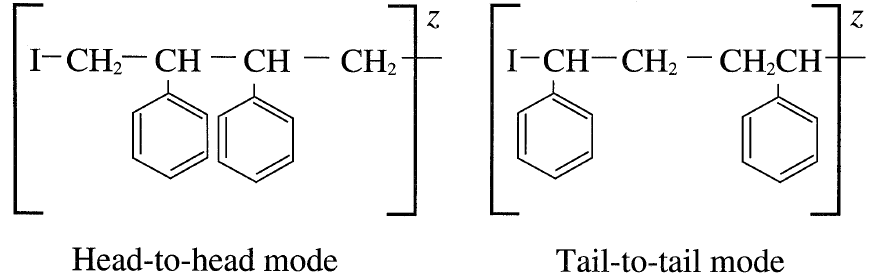
Chain growth continues until the propagating polymer chain terminates.
 الاكثر قراءة في البترو كيمياويات
الاكثر قراءة في البترو كيمياويات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


