

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
COMPOSITION OF CRUDE OILS
المؤلف:
sami matar & Lewis. F. Hatch
المصدر:
Chemistry of PETROCHEMICAL PROCESSES
الجزء والصفحة:
p12
13-2-2016
5231
COMPOSITION OF CRUDE OILS
The Hydrocarbon
The principal constituents of most crude oils are hydrocarbon compounds.
All hydrocarbon classes are present in the crude mixture, except alkenes and alkynes. This may indicate that crude oils originated under a reducing hydrocarbon classes found in all crude oils. atmosphere. The following is a brief description of the different
Alkanes (Paraffins)
Alkanes are saturated hydrocarbons having the general formula CnH2n+2. The simplest alkane, methane (CH4), is the principal constituent of natural gas. Methane, ethane, propane, and butane are gaseous hydrocarbons at ambient temperatures and atmospheric pressure. They are usually found associated with crude oils in a dissolved state.
Normal alkanes (n-alkanes, n-paraffins) are straight-chain hydrocarbons having no branches. Branched alkanes are saturated hydrocarbons with an alkyl substituent or a side branch from the main chain. Abranched alkane with the same number of carbons and hydrogens as an n-alkane is called an isomer. For example, butane (C4H10) has two isomers, n-butane and 2-methyl propane (isobutane). As the molecular weight of the hydrocarbon increases, the number of isomers also increases. Pentane (C5C12) has three isomers; hexane (C6H14) has five. The following shows the isomers of hexane:
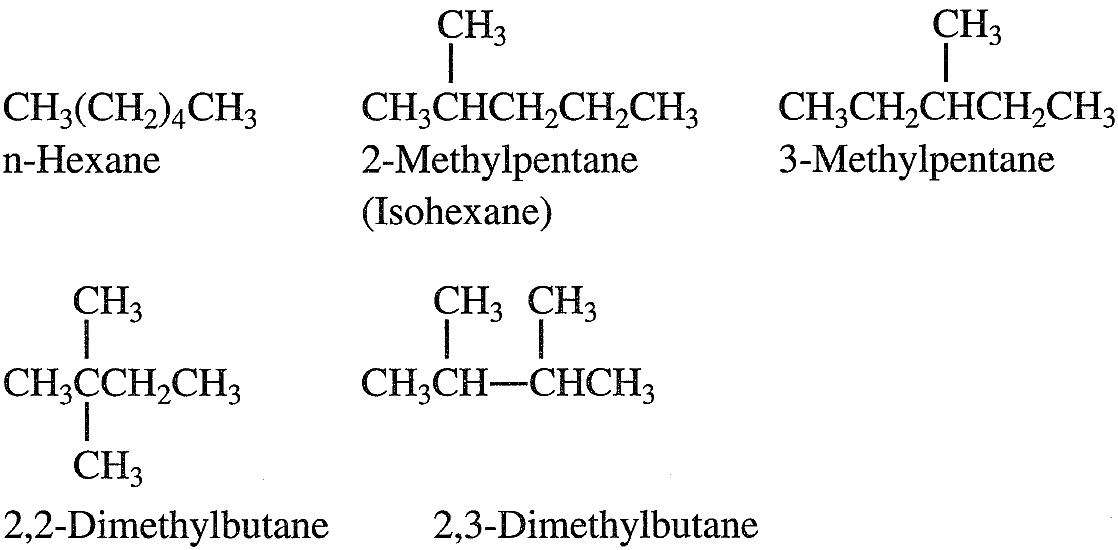
An isoparaffin is an isomer having a methyl group branching from carbon number 2 of the main chain. Crude oils contain many short, medium, and long-chain normal and branched paraffins. A naphtha fraction (obtained as a light liquid stream from crude fractionation) with a narrow boiling range may contain a limited but still large number of isomers. Cycloparaffins (Naphthenes)
Saturated cyclic hydrocarbons, normally known as naphthenes, are also part of the hydrocarbon constituents of crude oils. Their ratio, however, depends on the crude type. The lower members of naphthenes are cyclopentane, cyclohexane, and their mono-substituted compounds.
They are normally present in the light and the heavy naphtha fractions.
Cyclohexanes, substituted cyclopentanes, and substituted cyclohexanes are important precursors for aromatic hydrocarbons.
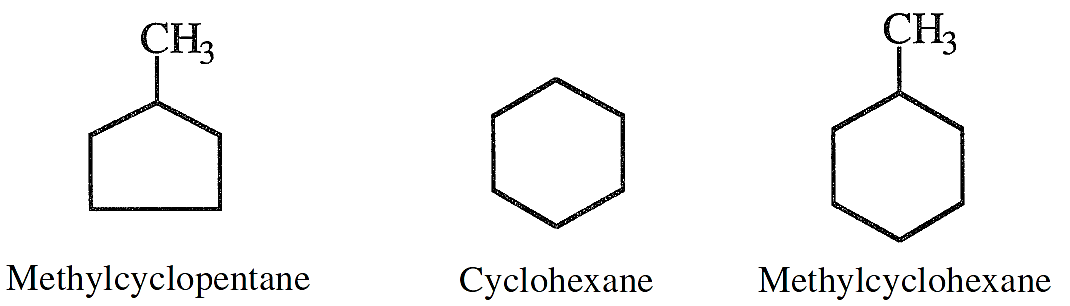
The examples shown here are for three naphthenes of special importance. If a naphtha fraction contains these compounds, the first two can be converted to benzene, and the last compound can dehydrogenate to toluene during processing. Dimethylcyclohexanes are also important precursors for xylenes.
Heavier petroleum fractions such as kerosine and gas oil may contain two or more cyclohexane rings fused through two vicinal carbons.
Aromatic Compounds
Lower members of aromatic compounds are present in small amounts in crude oils and light petroleum fractions. The simplest mononuclear aromatic compound is benzene (C6H6). Toluene (C7H8) and xylene (C8H10) are also mononuclear aromatic compounds found in variable amounts in crude oils. Benzene, toluene, and xylenes (BTX) are important petrochemical intermediates as well as valuable gasoline components.
Separating BTX aromatics from crude oil distillates is not feasible because they are present in low concentrations. Enriching a naphtha fraction with these aromatics is possible through a catalytic reforming process. Binuclear aromatic hydrocarbons are found in heavier fractions than naphtha. Trinuclear and polynuclear aromatic hydrocarbons, in combination with heterocyclic compounds, are major constituents of heavy crudes and crude residues. Asphaltenes are a complex mixture of aromatic and heterocyclic compounds. The nature and structure of some of these compounds have been investigated. The following are representative examples of some aromatic compounds found in crude oils:
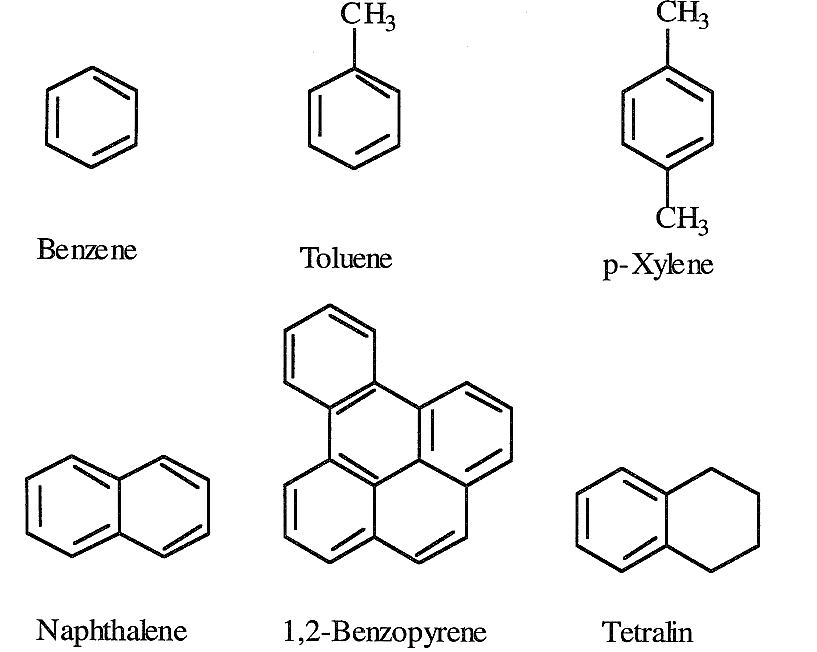
Only a few aromatic-cycloparaffin compounds have been isolated and identified. Tetralin is an example of this class.
 الاكثر قراءة في البترو كيمياويات
الاكثر قراءة في البترو كيمياويات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















