

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
OXO ALDEHYDES AND ALCOHOLS (Hydroformylation Reaction)
المؤلف:
sami matar & Lewis. F. Hatch
المصدر:
Chemistry of PETROCHEMICAL PROCESSES
الجزء والصفحة:
p 164
16-8-2017
2161
OXO ALDEHYDES AND ALCOHOLS (Hydroformylation Reaction)
Hydroformylation of olefins (Oxo reaction) produces aldehydes with one more carbon than the reacting olefin. For example, when ethylene is used, propionaldehyde is produced. This reaction is especially important for the production of higher aldehydes that are further hydrogenated to the corresponding alcohols. The reaction is catalyzed with cobalt or rhodium complexes. Olefins with terminal double bonds are more reactive and produce aldehydes which are hydrogenated to the corresponding primary alcohols. With olefins other than ethylene, the hydroformylation reaction mainly produces a straight chain aldehyde with variable amounts of branched chain aldehydes. The reaction could be generally represented as:
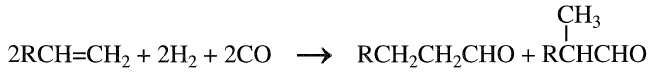
The largest commercial process is the hydroformylation of propene, which yields n-butyraldehyde and isobutyraldehyde. n-Butyraldehyde (n butanal) is either hydrogenated to n-butanol or transformed to 2- ethyl-hexanol via aldol condensation and subsequent hydrogenation. 2- Ethylhexanol is an important plasticizer for polyvinyl chloride.
Other olefins applied in the hydroformylation process with subsequent hydrogenation are propylene trimer and tetramer for the production of decyl and tridecyl alcohols, respectively, and C7 olefins (from copolymers of C3 and C4 olefins) for isodecyl alcohol production.
Several commercial processes are currently operative. Some use a rhodium catalyst complex incorporating phosphine ligands HRhCO(PPh3)2 at relatively lower temperatures and pressures and produce less branched aldehydes. Older processes use a cobalt carbonyl complex HCo(CO)4 at higher pressures and temperatures and produce a higher ratio of the branched aldehydes. The hydroformylation reaction using phosphine ligands occurs in an aqueous medium. A higher catalyst activity is anticipated in aqueous media than in hydrocarbons. Selectivity is also higher. Having more than one phase allows for complete separation of the catalyst and the products.
In order to make the catalysts soluble in water, ionic ligands are attached to the catalyst. The Rhurchemie/Rhone-Poulenc process for the production of butyraldehyde from propylene is based on this technology.
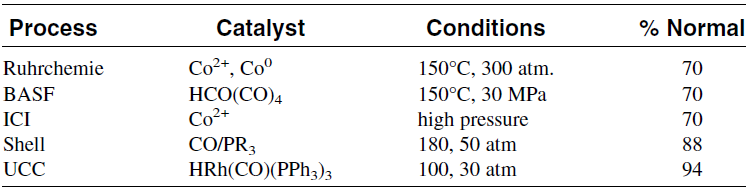
Hydroformylation of higher olefins using ionic phosphine catalysts that are solubilized in both reactants and products was investigated by Union Carbide researchers. This yields a one-phase homogeneous system. The catalyst is recovered outside the reaction zone. Although this is a single-phase system, these catalysts could be induced to separate into a nonpolar product and polar catalyst phases. This technology provides an effective means of catalyst recovery. Cobalt catalysts have also been investigated. Hoechest researchers have developed a water soluble cobalt cluster compound that can hydroformylate olefins in a two-phase system. Hydroformylation of higher olefins is possible when polyethylene glycol is used as a solvent. Higher olefins have greater affinity for ethylene glycol than for water, therefore allowing greater reaction rates. To facilitate the separation of the products, pentane is added to the system. The reaction takes place at 120°C and 70 KPa. When 1-hexene is used, the ratio of n-heptanal to the iso- was 0.73–3.75.33 Table 1.1 shows the hydroformylation conditions of some commercial processes.
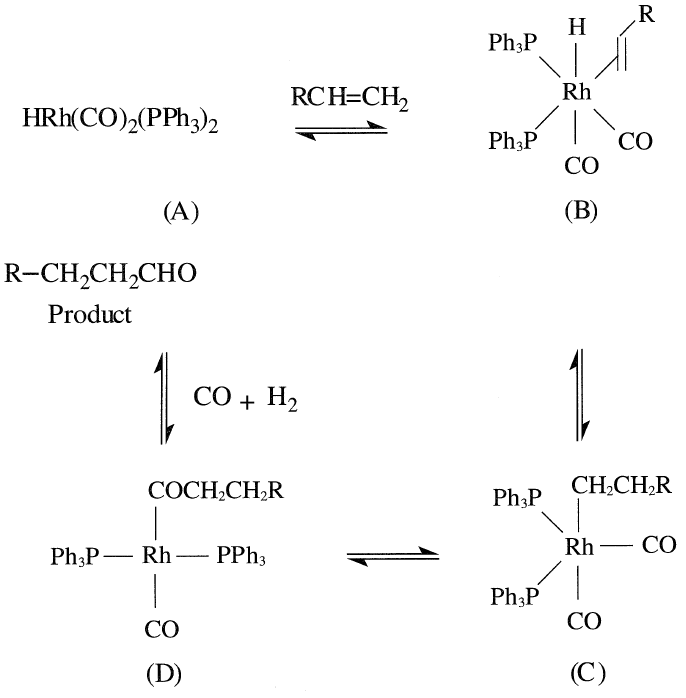
A simplified mechanism for the hydroformylation reaction using the rhodium complex starts by the addition of the olefin to the catalyst (A) to form complex (B). The latter rearranges, probably through a fourcentered intermediate, to the alkyl complex (C). A carbon monoxide insertion gives the square-planar complex (D). Successive H2 and CO addition produces the original catalyst and the product:
Table 1.1: Catalysts used in some commerical oxo processes and approximate conditions for propylene hydroformylation


PPh3 is triphenyl phosphine.
 الاكثر قراءة في البترو كيمياويات
الاكثر قراءة في البترو كيمياويات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















