


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 25-2-2018
Date: 25-2-2018
Date: 19-4-2017
|
Solubility
Although solvents may form two visibly distinct phases when mixed together, they are often somewhat soluble in each other and will, in fact, become mutually saturated when mixed with each other. Data on the solubility of various solvents in water (Table 1.1) and on the solubility of water in other solvents (Table 1.2) should be consulted when selecting an extraction solvent pair. For example, 1.6% of the solvent dichloromethane (or methylene chloride) is soluble in water. Conversely, water is 0.24% soluble in dichloromethane. According to Table 1.2, when the phases are separated for recovery of the extracted analyte, the organic solvent layer will contain water.
Table 1.1. Solubility in Water
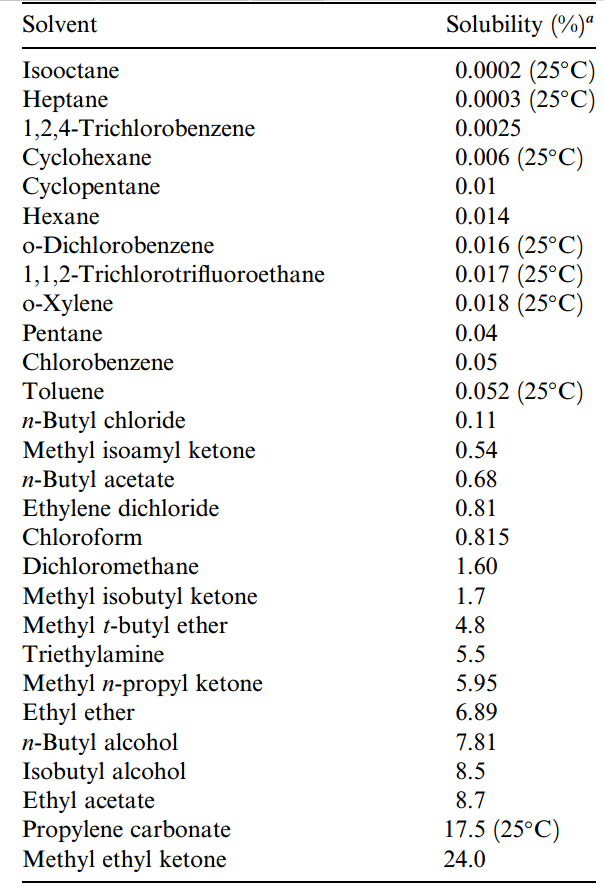
Similarly, according to Table 1.1, after extraction the depleted aqueous phase will be saturated with organic solvent and may pose a disposal problem. (Author’s note:I previously recounted my LLE experience with disposal of extracted aqueous samples that were cleaned of pesticide residues but saturated with diethyl ether. Diethyl ether is 6.89% soluble in water at 20º C.)
Table 1.2. Solubility of Water in Each Solvent
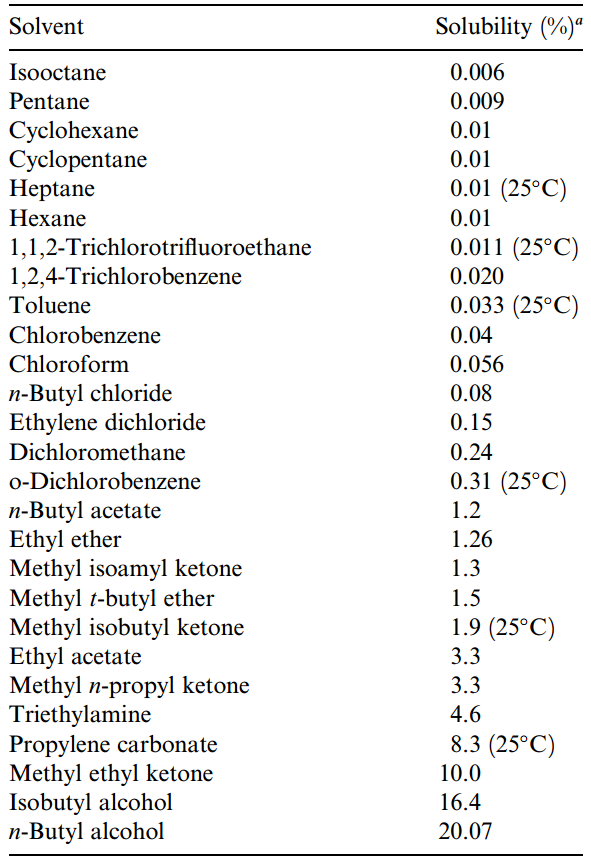



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|