


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 12-1-2017
Date: 22-3-2017
Date: 22-3-2017
|
Germides, stannides and plumbides
Germanium, tin and lead do not form solid state binary compounds with metals. In contrast, the formation of Zintl phases and Zintl ions which contain clusters of group 14metal atoms, is characteristic of these elements. As we have already seen, anionic units containing silicon are known, in addition to the formation of metal silicides with extended solid state structures. The synthesis of [Sn5]2- typifies the preparations of other Zintl ions and the use of the encapsulating ligand crypt-222 to bind an alkali metal counter-ion has played a crucial role in the development of Zintl ion chemistry. Thus, salts such as [K(crypt-222)]2[Sn5] and [Na(crypt-222)]4[Sn9] can be isolated. Modern technology allows low-temperature X-ray diffraction studies of sensitive (e.g. thermally unstable) compounds. It is now‡ therefore possible to investigate salts such as [Li(NH3)4]4[Pb9]:NH3 and [Li(NH3)4]4[Sn9]:NH3 which are formed by the direct reaction of an excess of Pb or Sn in solutions of lithium in liquid NH3. Diamagnetic Zintl ions include [M4]4- (M¼Ge, Sn, Pb), [M5]2- (M=Sn, Pb), [M9]4- (M=Ge, Sn, Pb), [Ge9]2-, [Ge10]2-, [Sn8Tl]3-, [Sn9Tl]3- and [Pb2Sb2]2-. Paramagnetic ions are exemplified by [Sn9]3- and [Ge9]3-.
Figure 1.1 shows the structures of [Sn9]4- and [Ge9]3-, and illustrates some of the main deltahedral families of the group 14 Zintl ions. Bonding in these ions is delocalized, and for the diamagnetic clusters, Wade’s rules can be used to rationalize the observed structures. Wade’s rules were developed for borane clusters. A {BH}-unit contributes two electrons to cluster bonding and, similarly, a group 14 atom contributes two electrons to cluster bonding if a lone pair of electrons is localized outside the cage. Thus, in bonding terms, an Si, Ge, Sn or Pb atom can mimic a {BH}-unit. More strictly, an atom of each group 14 element is isolobal with a {BH}-unit.
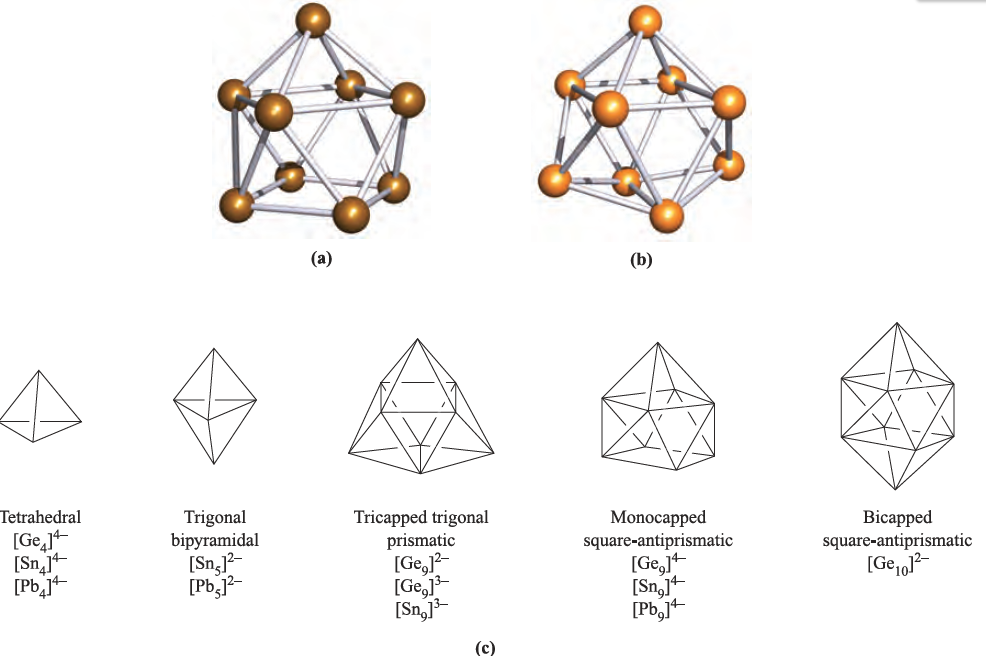
Fig. 1.1 The structures, established by X-ray diffraction, of (a) [Sn9]4_, determined for the salt [Na(crypt-222)]4[Sn9] [J.D. Corbett et al. (1977) J. Am. Chem. Soc., vol. 99, p. 3313], and (b) [Ge9]3-, determined for the compound [K(crypt-222)]3[Ge9].PPh3 [C. Belin et al. (1991) New J. Chem., vol. 15, p. 931]; for discussion of cryptand ligands including crypt-222. (c) Schematic representations of structure types for selected Zintl ions.
Thus, [Sn9]4- has 11 pairs of electrons with which to bond nine Sn atoms. This means that there are (n + 2) pairs of electrons for n vertices, and so [Sn9]4- is a nido-cage, based on a 10-vertex deltahedron with one vertex vacant. This corresponds to the observed structure of a monocapped square-antiprism.
Reaction conditions are critical to the selective formation of a Zintl ion. The alloy KSn2 reacts with crypt-222 in 1,2-diaminoethane to give [K(crypt-222)]3[Sn9] containing the paramagnetic [Sn9]3- ion.
However, reaction times must be less than two days, since longer periods favour the formation of [K(crypt-222)]4[Sn9] containing the diamagnetic [Sn9]4- ion. The paramagnetic clusters [Sn9]3- and [Ge9]3- both adopt distorted tricapped trigonal prismatic structures (Figure 1.1b). When Cs2K[Ge9] is added to a mixture of 1,2-ethanediamine and crypt-222, coupling of the [Ge9]3- radicals occurs to give Cs4[K(crypt-222)]2[(Ge9)2]; formally, the coupling involves the oxidation of one lone pair on each [Ge9]3-cage. The structure of the [(Ge9)2]6- ion (Figure 1.2a) consists of two monocapped square-antiprismatic clusters (each with delocalized bonding) connected by a localized, two-centre two-electron Ge_Ge bond. Wade’s rules can be applied to each cage in [(Ge9)2]6- as follows:
The Zintl ions shown in Figure 1.1 are closo- or nidoclusters. The compounds Rb4Li2Sn8 and K4Li2Sn8, which contain arachno-[Sn8]6- (Figure 1.2b), have been prepared by the direct fusion of tin metal with the respective alkali metals.
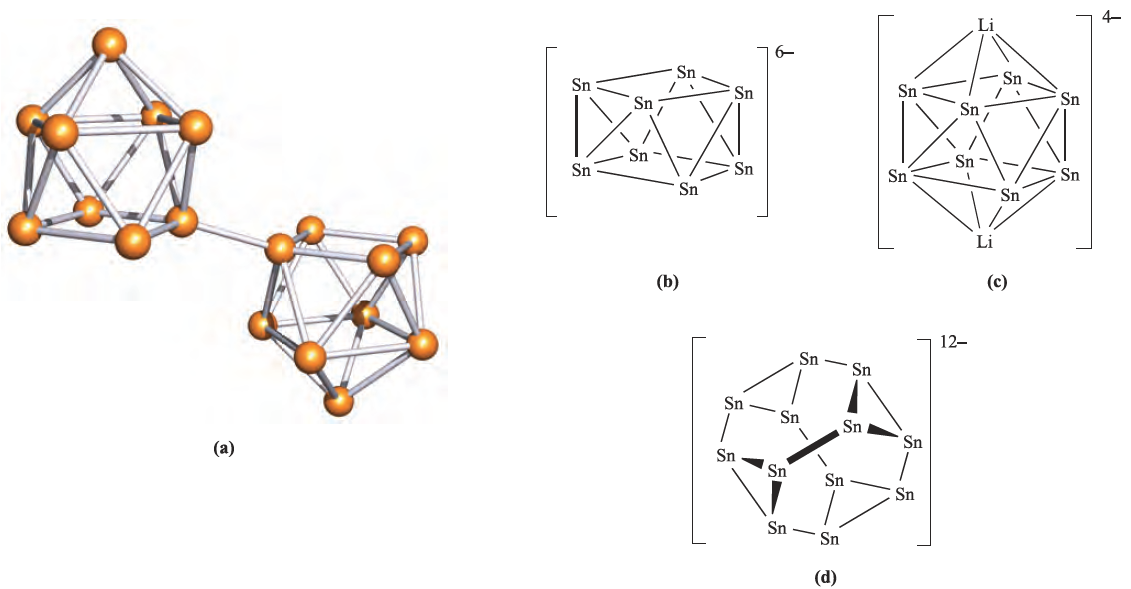
Fig. 1.2 (a) The structure (X-ray diffraction) of the [(Ge9)2]6- ion in Cs4[K(crypt-222)]2[(Ge9)2]. 6en (en=1,2- ethanediamine) [L. Xu et al. (1999) J. Am. Chem. Soc., vol. 121, p. 9245]. (b) The arachno-[Sn8]6- cluster in Rb4Li2Sn8. (c) The solid state structure of Rb4Li2Sn8 shows that Li ions cap the open cage to give [Li2Sn8]4- (see text). (d) The open [Sn12]12- cluster in the compound CaNa10Sn12; the cage encapsulates a Ca+2 ion.
X-ray diffraction studies on Rb4Li2Sn8 show that the arachno-[Sn8]6- cluster is stabilized by interactions with Li ions which effectively close up the open cage as shown in Figure 1.2c. In addition, each Li ion interacts with an Sn_Sn edge of an adjacent cluster and as a result, a network of interconnected cages is formed, with Rb ions in cavities between the Zintl ions. The combination of small and large cations is an important factor in the stabilization of this system. The same strategy has been used to stabilize another open-cage Zintl ion, [Sn12]12- (Figure 1.2d), which is formed by fusing together stoichiometric amounts of Na, Ca and Sn. The product is CaNa10Sn12, and in the solid state, the Ca2+ ion provides a stabilizing effect by being sited at the centre of the [Sn12]12- cluster. A related system in which Sr2+ replaces Ca2+ has also been prepared. As more Zintl ions are isolated, challenges to the rationalization of the bonding within Wade’s rules are encountered.
For example, the oxidation of [Ge9]4- using PPh3, AsPh3, As or Sb gives [(Ge9)3]6-. The [(Ge9)3]6- anion (Figure 1.3) consists of three tricapped trigonal prismatic cages, each with two elongated prism edges.
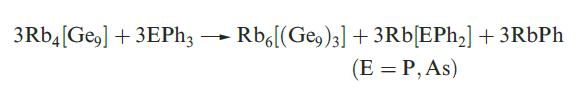 )1.1(
)1.1(
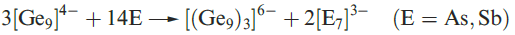 (1.2)
(1.2)
Outward-pointing radial orbitals on each B atom are involved in the formation of the external (exo) B_H π- bonds. Similarly, in most Zintl ions, the lone pair of electrons that is localized on each atom is accommodated in an outward-pointing orbital. In the oxidative coupling of two [Ge9]3- cages to give [(Ge9)2]6- (Figure 1.2a), the localized single bond that joins the cages and which formally arises from the oxidation of a lone pair per cluster is radially oriented with respect to each cluster. However, in [(Ge9)3]6- (Figure 1.3), the intercluster bonds are not radially related to each cluster, but lie parallel to the prism edges. In addition, the Ge_Ge bond lengths for the intercluster bonds are significantly longer in [(Ge9)3]6- than that in [(Ge9)2]6-. This suggests that the bonds that connect the cages in [(Ge9)3]6- are of bond orders less than 1 and that the bonding is not localized. It is, therefore, not possible to apply Wade’s rules to each cage in this tricluster system.
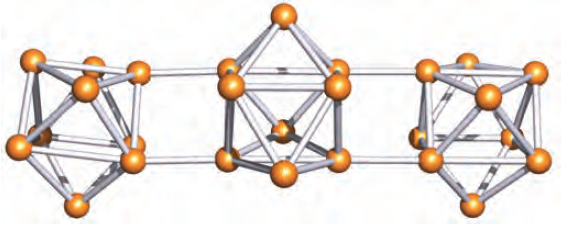
Fig. 1.3 The structure (X-ray diffraction) of the [(Ge9)3]6- ion in [Rb(crypt-222)]6[(Ge9)3].3en (en=1,2-ethanediamine) [A. Ugrinov et al. (2002) J. Am. Chem. Soc., vol. 124, p. 10990].



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|