
 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-10-2018
Date: 5-1-2019
Date: 7-12-2018
|
Deuterated compounds
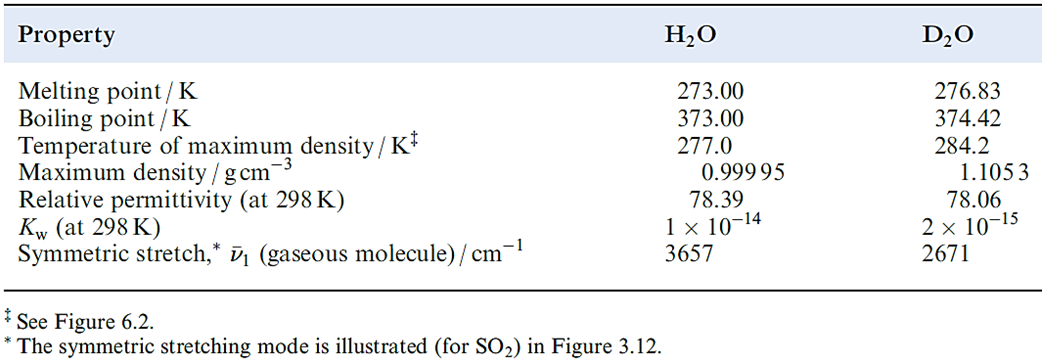
A deuterium label in heavy water is indicated by writing [2H2]water or water-d2, and similarly for other labelled compounds. Compounds in which H atoms have been replaced by D are used for a variety of purposes, e.g. as solvents in 1H NMR spectroscopy. In a fully deuterated material, the D-for-H exchange can have significant effects on the properties of the compound as is shown in Table 9.2 for H2O and D2O. The difference in boiling points indicates that intermolecular hydrogen bonding (see Sections 6.2 and 9.6) is stronger in D2O than in H2O. The major industrial use of D2O is as a moderator in nuclear reactors; D has a much lower cross-section for neutron capture than H, and D2O is a suitable material for reducing the energies of fast neutrons produced in fission without appreciably diminishing the neutron flux.
Table 9.2 Selected properties of H2O and D2O (‘heavy water’).
Many fully or partially deuterated compounds are available commercially, and the extent of deuterium labelling can be determined by mass spectrometry, density measurements (after conversion into water) or IR spectroscopy.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|