


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 13-2-2016
Date: 9-5-2016
Date: 10-9-2017
|
Acetone Production
Acetone (2-propanone), is produced from isopropanol by a dehydrogenation, oxidation, or a combined oxidation dehydrogenation route. The dehydrogenation reaction is carried out using either copper or zinc oxide catalyst at approximately 450–550°C. A 95% yield is obtained:

The direct oxidation of propylene with oxygen is a noncatalytic reaction occurring at approximately 90–140°C and 15–20 atmospheres. In this reaction hydrogen peroxide is coproduced with acetone. At 15% isopropanol conversion, the approximate yield of acetone is 93% and that for H2O2 is 87%:
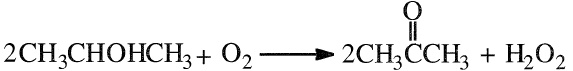
The oxidation process uses air as the oxidant over a silver or copper catalyst. The conditions are similar to those used for the dehydrogenation reaction.
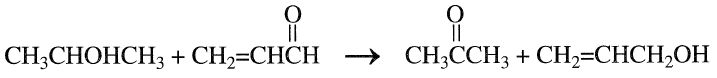
Acetone can also be coproduced with allyl alcohol in the reaction of acrolein with isopropanol. The reaction is catalyzed with an MgO and ZnO catalyst combination at approximately 400°C and one atmosphere.
It appears that the hydrogen produced from the dehydrogenation of isopropanol and adsorbed on the catalyst surface selectively hydrogenates the carbonyl group of acrolein:
A direct route for acetone from propylene was developed using a homogeneous catalyst similar to Wacker system (PdCl2/CuCl2). The reaction conditions are similar to those used for ethylene oxidation to acetaldehyde. Today, most acetone is obtained via a cumene hydroperoxide process where it is coproduced with phenol.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|