


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 22-8-2017
Date: 9-5-2016
Date: 13-2-2016
|
HYDROGEN CYANIDE (HCN)
Hydrogen cyanide (hydrocyanic acid) is a colorless liquid (b.p. 25.6°C) that is miscible with water, producing a weakly acidic solution.
It is a highly toxic compound, but a very useful chemical intermediate with high reactivity. It is used in the synthesis of acrylonitrile and adiponitrile, which are important monomers for plastic and synthetic fiber production. Hydrogen cyanide is produced via the Andrussaw process using ammonia and methane in presence of air. The reaction is exothermic, and the released heat is used to supplement the required catalyst-bed energy:
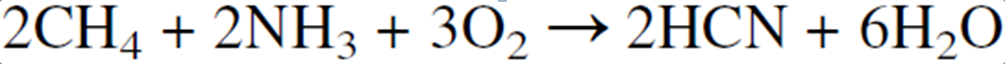
A platinum-rhodium alloy is used as a catalyst at 1100°C. Approximately equal amounts of ammonia and methane with 75 vol % air are introduced to the preheated reactor. The catalyst has several layers of wire gauze with a special mesh size (approximately 100 mesh).
The Degussa process, on the other hand, reacts ammonia with methane in absence of air using a platinum, aluminum-ruthenium alloy as a catalyst at approximately 1200°C. The reaction produces hydrogen cyanide and hydrogen, and the yield is over 90%. The reaction is endothermic and requires 251 KJ/mol.
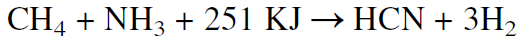
Hydrogen cyanide may also be produced by the reaction of ammonia and methanol in presence of oxygen:
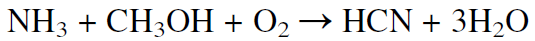
Hydrogen cyanide is a reactant in the production of acrylonitrile, methyl methacrylates (from acetone), adiponitrile, and sodium cyanide. It is also used to make oxamide, a long-lived fertilizer that releases nitrogen steadily over the vegetation period. Oxamide is produced by the reaction of hydrogen cyanide with water and oxygen using a copper nitrate catalyst at about 70°C and atmospheric pressure:




|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|