


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-8-2017
Date: 8-8-2017
Date: 5-9-2017
|
HYDROGEN
Hydrogen is the lightest known element. Although only found in the free state in trace amounts, it is the most abundant element in the universe and is present in a combined form with other elements. Water, natural gas, crude oils, hydrocarbons, and other organic fossil materials are major sources of hydrogen.
Hydrogen has been of great use to theoretical investigation. The structure of the atom developed by Bohr (Nobel Prize Winner 1922) was based on a model of the hydrogen atom. Chemically, hydrogen is a very reactive element. Obtaining hydrogen from its compounds is an energyextensive process. To decompose water into hydrogen and oxygen, an energy input equal to an enthalpy change of +286 KJ/mol is required :

Electrolysis, and thermochemical and photochemical decomposition of water followed by purification through diffusion methods are expensive processes to produce hydrogen.
The most economical way to produce hydrogen is by steam reforming petroleum fractions and natural gas (Figure 4-1). In this process, two major sources of hydrogen (water and hydrocarbons) are reacted to produce a mixture of carbon monoxide and hydrogen (synthesis gas).
Hydrogen can then be separated from the mixture after shift converting carbon monoxide to carbon dioxide. Carbon oxides are removed by passing the mixture through a pressure swing adsorption system. Recently, a new process has been developed to manufacture hydrogen by steam reforming methanol. In this process, an active catalyst is used to decompose methanol and shift convert carbon monoxide to carbon dioxide. The produced gas is cooled, and carbon dioxide is removed:
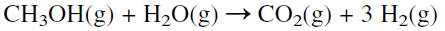
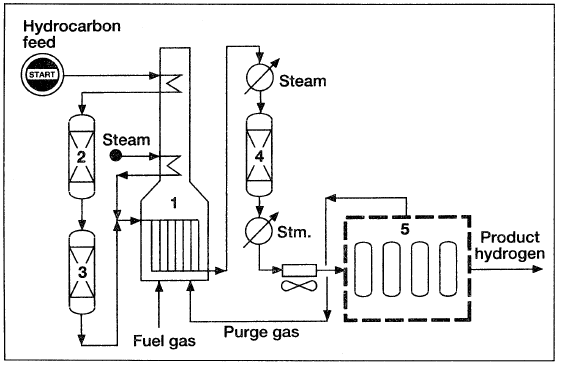
Figure 1.1. A process for producing hydrogen by steam reforming of hydrocarbons: (1) reforming furnace (2,3) purification section, (4) shift converter, (5) pressure swing adsorption.
This process is used to produce relatively small quantities (0.18–1.8 MMscfd) of highly pure hydrogen when methanol is available at a reasonable price. In the petroleum refining industry, hydrogen is essentially obtained from catalytic naphtha reforming, where it is a coproduct with reformed gasoline.
The use of hydrogen in the chemical and petroleum refining industries is of prime importance. Hydrogen is essentially a hydrogenating agent. For example, it is used with vegetable oils and fats to reduce unsaturated esters (triglycerides). It is also a reducing agent for sulfide ores such as zinc and iron sulfides (to get the metals from their ores).
Hydrogen use in the petroleum refining includes many processing schemes such as hydrocracking, hydrofinishing of lube oils, hydrodealkylation and hydrodesulfurization of petroleum fractions and residues. Hydrocracking of petroleum resids is becoming more important to produce lighter petroleum distillates of low sulfur and nitrogen content to meet stringent government-mandated product specifications to control pollution.
In the petrochemical field, hydrogen is used to hydrogenate benzene to cyclohexane and benzoic acid to cyclohexane carboxylic acid. These compounds are precursors for nylon production. It is also sed to selectively hydrogenate acetylene from C4 olefin mixture. As a constituent of synthesis gas, hydrogen is a precursor for ammonia, methanol, Oxo alcohols, and hydrocarbons from Fischer Tropsch processes. The direct use of hydrogen as a clean fuel for automobiles and buses is currently being evaluated compared to fuel cell vehicles that use hydrocarbon fuels which are converted through on-board reformers to a hydrogen-rich gas. Direct use of H2 provides greater efficiency and environmental benefits.
Due to the increasing demand for hydrogen, many separation techniques have been developed to recover it from purge streams vented from certain processing operations such as hydrocracking and hydrotreating.
In addition to hydrogen, these streams contain methane and other light hydrocarbon gases. Physical separation techniques such as adsorption, diffusion, and cryogenic phase separation are used to achieve this goal.
Adsorption is accomplished using a special solid that preferentially adsorbs hydrocarbon gases, not hydrogen. The adsorbed hydrocarbons are released by reducing the pressure. Cryogenic phase separation on the other hand, depends on the difference between the volatilities of the components at the low temperatures and high pressures used. The vapor phase is rich in hydrogen, and the liquid phase contains the hydrocarbons.
Hydrogen is separated from the vapor phase at high purity. Diffusion separation processes depend on the permeation rate for gas mixtures passing through a special membrane. The permeation rate is a function of the type of gas feed, the membrane material, and the operating conditions. Gases with smaller molecular sizes such as helium and hydrogen permeate membranes more readily than larger molecules such as methane and ethane.4 An example of membrane separator is the hollow fiber type shown in Figure 1.2. After the feed gas is preheated and filtered it enters the membrane separation section. This is made of a permeater vessel containing 12-inch diameter bundles (resemble filter cartridges) and consists of millions of hollow fibers. The gas mixture is distributed in the annulus between the fiber bundle and the vessel wall.
Hydrogen, being more permeable, diffuses through the wall of the hollow fiber and exits at a lower pressure. The less permeable hydrocarbons flow around the fiber walls to a perforated center tube and exit at approximately feed pressure. It has been reported that this system can deliver a reliable supply of 95+% pure hydrogen from off-gas streams having as low as 15% H2.
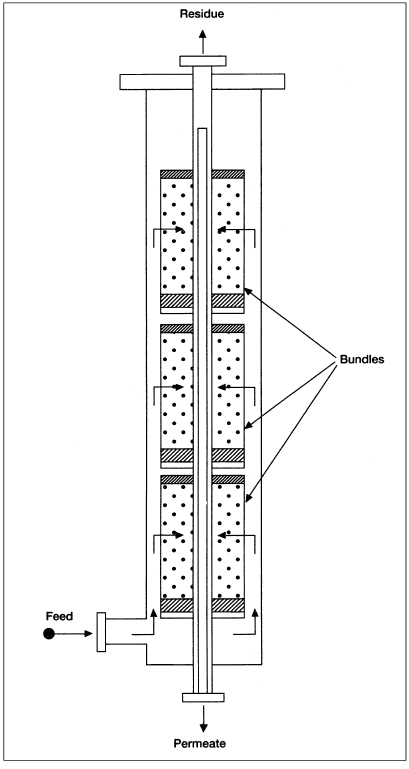
Figure 1.2. Permeator for gas separation



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|