


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 27-4-2019
Date: 27-6-2019
Date: 8-3-2019
|
Atomic Radii
The electron cloud around an atomic nucleus makes the concept of atomic size somewhat imprecise, but it is useful to refer to an atomic radius. One can arbitrarily divide the distance between centers of two bonded atoms to arrive at two radii, based on the crude picture that two bonded atoms are spheres in contact. If the bonding is covalent, the radius is called a covalent radius (see Table 8-2); if it is ionic, the radius is an ionic radius . The radius for non-bonded atoms may be defined in terms of the distance of closest non-bonding approach; such a measure is called the van der Waals radius. These three concepts of size are illustrated in Figure 1.1.
Generalizations regarding atomic size: (1) Within a column of the periodic table, radii increase with increasing atomic number, a result of the increasing value of n for the valence electrons. (2) Within a given period (row) of the table, covalent radii generally decrease with increasing Z; n is constant across the period, but the nuclear charge increases. (3) A cation radius is small compared with the covalent radius of the neutral atom since one or more valence electrons has been removed. (4) An anion radius is significantly greater than the covalent radius of the neutral atom since the extra electron(s) are held less tightly (more electrons, but the same nuclear charge).
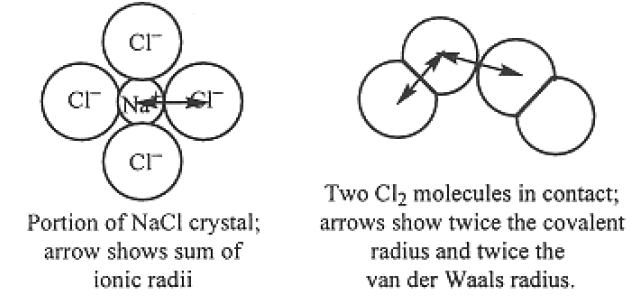
Figure 1.1. Illustrations of ionic, covalent, and van der Waals radii.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|