


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-3-2017
Date: 5-3-2019
Date: 27-4-2019
|
Sulfuric acid, H2SO4
Sulfuric acid is by far the most important of the oxoacids of sulfur and is manufactured on a huge scale by the Contact process. The first stages of this process (conversion of SO2 to SO3 and formation of oleum) were described in Section 15.8; the oleum is finally diluted with water to give H2SO4. Pure H2SO4 is a colourless liquid with a high viscosity caused by extensive intermolecular hydrogen bonding. Gas-phase H2SO4 molecules have C2 symmetry with S_O bond distances that reflect two different types of S_O bond. Diagram 1.1 shows a hypervalent structure for H2SO4, and 1.2 gives a bonding scheme in which the S atom obeys the octet rule (refer back to the discussion of bonding in Section 15.3). In the sulfate ion, all four S_O bond distances are equal (149 pm) because of charge delocalization, and in [HSO4]- , the S_OH bond distance is 156 pm and the remaining S_O bonds are of equal length (147pm).

(1.1) (1.2) (1.3)
In aqueous solution,H2SO4 acts as a strong acid (equation 1.1) but the [HSO4]- ion is a fairly weak acid (equation 1.2 and Table 15.8). Two series of salts are formed and can be isolated, e.g. KHSO4 and K2SO4.
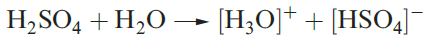 (1.1)
(1.1)
 (1.2)
(1.2)
Dilute aqueous H2SO4 (typically 2M) neutralizes bases (e.g. equation 1.3), and reacts with electropositive metals, liberating H2, and metal carbonates (equation 1.4).
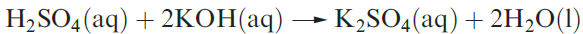 (1.3)
(1.3)
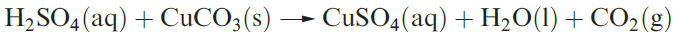 (1.4)
(1.4)
Commercial applications of sulfate salts are numerous, e.g. (NH4(2SO4 as a fertilizer, CuSO4 in fungicides, MgSO4 as a laxative, and hydrated CaSO4 uses of H2SO4 were included in Figure 15.3. Concentrated H2SO4 is a good oxidizing agent (e.g. reaction 15.88) and a powerful dehydrating agent (see Box 11.4); its reaction with HNO3 is important for organic nitrations (equation 1.5).
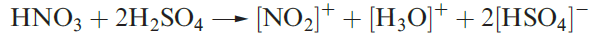 (1.4)
(1.4)
Commercial applications of sulfate salts are numerous, e.g.)NH4(2SO4 as a fertilizer, CuSO4 in fungicides, MgSO4 as a laxative, and hydrated CaSO4 uses of H2SO4 were included in Figure 15.3. Concentrated H2SO4 is a good oxidizing agent (e.g. reaction 15.88) and a powerful dehydrating agent; its reaction with HNO3 is important for organic nitrations (equation 1.5).
 (1.5)
(1.5)
Although HF/SbF5 is a superacid, attempts to use it to protonate pure H2SO4 are affected by the fact that pure sulfuric acid undergoes reaction 1.6 to a small extent. The presence of the [H3O] + ions in the HF/SbF5 system prevents complete conversion of H2SO4 to [H3SO4]+.
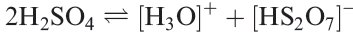 (1.6)
(1.6)
An ingenious method of preparing a salt of [H3SO4] is to use reaction 1.7 which is thermodynamically driven by the high Si_F bond enthalpy term in Me3SiF. In the solid state structure of [D3SO4][SbF6]- (made by using DF in place of HF), the cation has structure 1.3 and there are extensive O_D……F interactions between cations and anions.
 (1.7)
(1.7)



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|