
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-3-2017
Date: 29-3-2017
Date: 22-3-2017
|
Oxoacids and their salts Hypofluorous acid, HOF
Fluorine is unique among the halogens in forming no species in which it has a formal oxidation state other than _1. The only known oxoacid is hypofluorous acid, HOF, which is unstable and does not ionize in water but reacts according to equation 1.1; no salts are known. It is obtained by passing F2 over ice at 230K (equation 1.2) and condensing the gas produced. At 298 K, HOF decomposes rapidly (equation 1.3).
 (1.1)
(1.1)
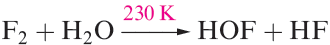 (1.2)
(1.2)
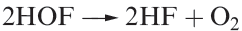 (1.3)
(1.3)



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
دراسة تستعرض آلام السجناء السياسيين في حقبة البعث المجرم في العراق
|
|
|