


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-6-2020
Date: 14-5-2020
Date: 7-2-2018
|
Constants: Comparing amounts of products and reactants
Sometimes there’s a lot of product (chemical species on the right-hand side of the double arrow) when the reaction reaches equilibrium, and sometimes there’s very little. You can tell the relative amounts of reactants and products at equilibrium if you know the equilibrium constant for the reaction. Look at a hypothetical equilibrium reaction:
aA + bB ↔ cC + dD
The capital letters stand for the chemical species, and the small letters represent the coefficients in the balanced chemical equation. The equilibrium constant (represented as Keq) is mathematically defined as
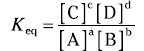
The numerator contains the product of the two chemical species on the right-hand side of the equation, with each chemical species raised to the power of its coefficient in the balanced chemical equation. The denominator is the same, but you use the chemical species on the left-hand side of the equation. Note that sometimes chemists use the Kc notation instead of the Keq form.
The numerical value of the equilibrium constant gives you a clue about the relative amounts of products and reactants. The larger the value of the equilibrium constant (Keq), the more products are present at equilibrium. If, for example, you have a reaction that has an equilibrium constant of 0.001 at room temperature and 0.1 at 100°C, you can say that you’ll have much more product at the higher temperature.
Now I happen to know that the Keq for the Haber proces (the ammonia synthesis) is 3.5 × 108 at room temperature. This large value indicates that, at equilibrium, there’s a lot of ammonia produced from the nitrogen and hydrogen, but there’s still hydrogen and nitrogen left at equilibrium.



|
|
|
|
دراسة: حفنة من الجوز يوميا تحميك من سرطان القولون
|
|
|
|
|
|
|
تنشيط أول مفاعل ملح منصهر يستعمل الثوريوم في العالم.. سباق "الأرنب والسلحفاة"
|
|
|
|
|
|
|
لتعزيز التواصل مع الزائرات الأجنبيات : العتبة العلويّة المقدّسة تُطلق دورة لتعليم اللغة الإنجليزية لخادمات القسم النسويّ
|
|
|