


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 16-10-2016
Date: 28-10-2015
Date: 27-10-2016
|
Soils and Mineral Availability
Soils are derived from rock by processes of weathering. The initial rock may be volcanic (granite, basalt), metamorphosed (marble, slate), sedimentary (sandstone, limestone), or other types, but two things are important: Rock has a crystalline structure, and trapped within the structure are numerous types of contaminating ions and elements (Fig. 1). its crystal matrix prevents rock from being a suitable substrate for plant growth; it may contain most essential elements, but as long as they are part of the matrix or trapped by it, they cannot be absorbed and used by plants. Also, any water held by the rock as part of the crystal structure is unavailable to plants.
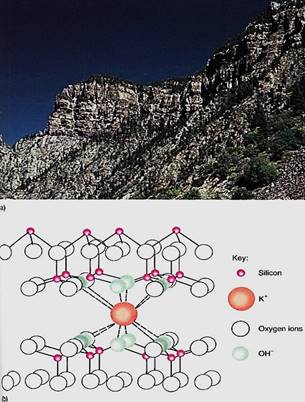
FIGURE 1: (a) Rock is a complex, highly contaminated crystal; as it weathers, it gradually breaks down into soil. (b) Within the crystal matrix of rock, numerous atoms of many different elements occur. As rock weathers, it breaks apart and atoms at the surface are liberated into the soil solution. This is potassium trapped in vermiculite, a type of clay
Two fundamental processes of weathering convert rock to soil: physical weathering and chemical weathering. As its name implies, physical weathering is the breakdown of rock by physical forces such as wind, water movement, and temperature changes. Ice is an important agent. During winter days, water from rain or melted snow seeps into capillary spaces within rock; at night, when temperatures fall below freezing, the expansion of wateras it becomes ice causes cracks to widen. Portions of rock, ranging from small flakes to large pieces, can be broken off. This is a slow process, but gradually the average particle size of the rock is reduced.
Runoff from rainstorms, avalanches, and similar forces wash rock fragments and pebbles into streams and rivers, where physical weathering accelerates. In rapidly moving streams the rocks are scoured by suspended sand grains; with high flow rates, rocks and boulders are carried downstream, grinding against each other and the stream bed. Wind-blown sand is a powerful erosive force, as are glaciers, which are extensive during the periodic ice ages.
Physical weathering produces a variety of sizes of soil particles; the largest ones that are technically important to soil are grains of coarse sand with a size range of 2.0 mm to 2mm. Particles only one tenth this large (0.2 to 0.02 mm) are fine sand, and those one tenth of this (0.02 to 0.002 mm) are silt. The finest particles, smaller than 0.002 mm in diameter, are clay particles, technically known as micelles (Fig. 2).
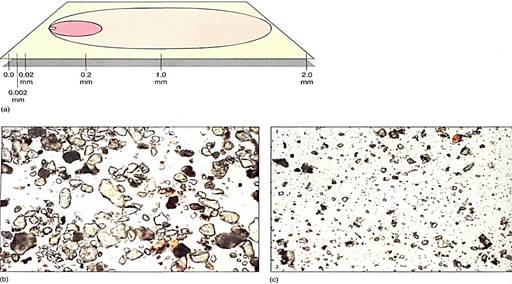
FIGURE 2: (a) Relative sizes of soil particles: From 0.2 to 2.0 mm is coarse sand, the next smaller is line sand, and so on. It is easy to calculate the amount of surface area, the volume of rock, and the volume of air/water space in a soil composed of only one type of particle. Assume you have 1 cm3 of pure coarse sand; the number of particles present is calculated by dividing 1 cm' by the volume of a cube measuring 2.0 mm on each side (the volume of the particle plus its surrounding air/water space). Next, multiply the number of particles by the volume of a sphere with a radius of 1.0 mm (each sphere has a diameter of 2.0 mm). This gives the total volume of rock in 1 cm3 of soil. Subtract this from 1 cm3 to obtain the volume of air or water that can be held by the soil. Finally, calculate the surface area of each sphere and multiply that by the number of particles to obtain HE total surface area that is releasing minerals into the 1 cm3 of soil. Now do this for fine sand, silt, and clay. Which soil has the most surface area? The greatest amount of air and water? (b) Panicles of fine sand, viewed by light microscopy (X 60). (c) Particles of silt and micelles of clay (X 60).
The various sizes produced by physical weathering are important factors in soil texture and porosity. In sands, particles are large and fit together poorly, so a great deal of space remains between particles. The spaces permit rapid gas diffusion, and roots in sandy soils typically are never starved for oxygen. During rain, the spaces fill with water, but typically they cannot hold it against gravity because the spaces are too broad to act as capillary tubes. Once rain slops, most of the water percolates downward and enters aquifers, flowing underground to wells, springs, and streams. Water that remains in the soil is held by capillary adhesion/cohesion(Fig. 3) and is said to be the field capacity of the soil. Much of this water is available to roots.
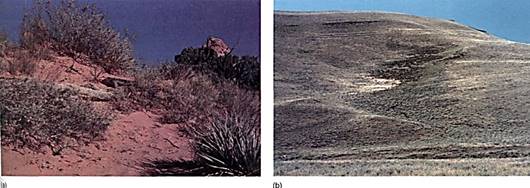
FIGURE 3: (a) This sandy soil has such large spaces between its particles that it cannot hold water well; it dries quickly after a ram. It has a low field capacity. (b) As water is pulled away from soil by gravity, it percolates downslope into valleys. Soil at the bottom of ravines has water available longer than soil on the sides of a slope; consequently, more vegetation grows at the bottom of a valley than on the sides.
Chemical weathering involves chemical reactions, and the most important agents are acids produced by decaying bodies, especially those of plants and fungi. In addition, many organisms secrete acids while alive, and the carbon dioxide produced during respiration can combine with water, forming carbonic acid. When an acid dissolves in water, it dissociates into a proton and an anion, both of which interact chemically with rock's crystal matrix and with embedded contaminant elements. In regions with a great deal of warmth, moisture, and abundant decaying vegetation, such as tropical regions, chemical weathering can be extremely rapid, and thick soils accumulate in a short time. In drier regions with long, cold winters and less vegetation, such as temperate mountains and the prairies of the plains states and Canada, fewer acids are available and chemical weathering is slower. It may take as long as 10 million years for just 1 cm of soil to form. Chemical weathering is j greatly increased if rock has already been reduced to sands and silts by physical weathering; these have a large surface area for the acids to attack.
Chemical weathering decreases soil particle size, but more importantly, it alters soil chemistry. As the crystal matrix dissolves, matrix elements become available to the plant and trapped elements are liberated. As the matrix breaks down, positively charged cations are freed; thus, the residual undissolved matrix has a negative charge (Fig.4). With coarse particles such as sand and silt, the surface-to-volume ratio is so small that the charge is not important. But with clay micelles, the total amount of surface per unit of soil volume is great. Because the particles have a negative charge, cations such as K+, Ca2+, Mg2+, and Cu2+ are held near the particles' surfaces. The bonding is much weaker than that of the festal matrix, so roots can absorb the cations. This attraction to the micelle surface is beneficial; without it, many important cations would be washed deep into the soil by passing rainwater.
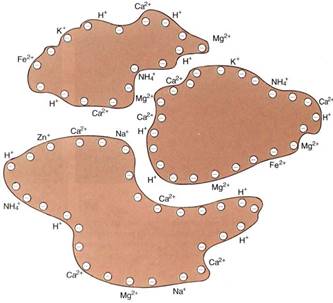
FIGURE 4: As rock weathers, the negatively charged components are most resistant, so the rock fragments (micelles) have a negative surface charge. Because of this, the cations that are released by weathering do not completely leave the rock, but are held by very weak electrical attraction.
CATION EXCHANGE
Because cations are loosely bound to micelle surfaces owing to their charge, roots cannot absorb them directly. Instead, the cations must first be freely dissolved in the soil solution; this is done by cation exchange. Roots and root hairs respire, giving off carbon dioxide. As this dissolves in the soil solution, some reacts chemically with water, forming carbonic acid H2CO3. This breaks down into a proton and a bicarbonate ion (Fig. 5), which can filter dissociate into a second proton and carbonate ion. The presence of protons acidifies the soil solution adjacent to roots and root hairs; as the protons diffuse, they bounce dose to a bound cation at a micelle surface. The presence of the proton's positive charge disrupts the electrical attraction of the cation, liberating it and trapping the proton. Because the proton was derived from waste carbon dioxide, its loss does not hurt the plant. The liberated cation may diffuse in the direction of the root and be absorbed and transported upward by xylem, or it may diffuse away from the root or strike another micelle, liberating either a proton or another cation. Over time, however, large quantities of cations are absorbed. Acidity caused by secretion of acids by bacteria and fungi, by the decomposition of humus, and by acid rain also result in the liberation of cations.
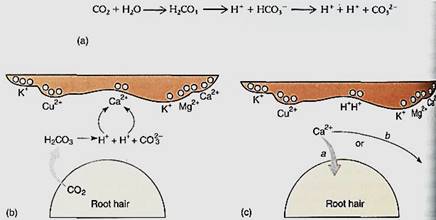
FIGURE 5 (a) The reaction of water and carbon dioxide results in carbonic acid (H2CO3), most of which dissociates into a proton and the bicarbonate anion. Some of the bicarbonate dissociates further, releasing another proton and the carbonate anion. (b) Protons from carbonic acid may diffuse close enough to a cation to disrupt its attraction to a soil micelle, liberating it (c). As the cation then diffuses through the soil, it may encounter a root (arrow b) or it may not (arrow c).
Soil particles are not the only structures that hold cations. Much of the decaying organic matter also forms negatively charged matrixes. The cellulose crystals of cell walls in mulch and humus are especially valuable not only for holding cations liberated from rock weathering but also for retaining the essential elements released by decaying protoplasm. Such organic matter also holds water and greatly improves the quality of any type of soil.
SOIL ACIDITY
Soil pH, the concentration of free protons in the soil solution, is important for cation exchange and the retention of cations in the soil during heavy rain. As acidity increases (pH becomes lower), the greater concentration of protons causes more cations to be released form soil micelles; these may be absorbed by roots or washed away in ground water. An extremely acid soil (pH of 4.0 to 5.0) tends to lose cations too rapidly and becomes a relatively poor soil. On the other hand, highly alkaline soils (pH of 9.0 to 10.0), which are frequent in dry climates, have too few protons to allow cation release, and concentrations of minerals can become excessively high.
Soil pH affects the chemical form of certain elements, causing them to change solubility. In acidic soils, aluminum and manganese can become so soluble as to reach toxic levels. In alkaline soils, iron and zinc become quite insoluble and unavailable to plants, but molybdenum is more soluble at a high pH. In general, a pH between 6.5 and 7.0 is best for many elements, especially iron, zinc, and phosphorus.
Many factors affect soil acidity, such as the chemical nature of the original rock, but probably the most important factor is rainfall. With high rainfall, there tends to be an abundance of vegetation that produces acids by means of respiration, excretion, and decay. With low rainfall, not only is there little vegetation, but there may not be any washing out of the soil. Cations build up, increasing soil alkalinity by increasing the concentration of hydroxyl ions. Just as dissociation of an acid produces a proton and an anion, dissociation of a base produces a hydroxyl and a cation:
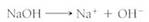
Because some soils are more acidic than others, plants have adapted to differences in the availability of essential elements (Table ). Natural selection favors mutations that Aw desert plants to cope with alkaline soils, whereas plants of wet areas must become adapted to acid soils. For example, azaleas, camellias, gardenias, and rhododendrons absolutely must have acidic soil; if planted into alkaline soils, they show stress symptoms immediately, often suffering from lack of iron as well as general poor health (Fig. 6).


FIGURE 6:This azalea leaf is from a plant growing in alkaline soil. Azaleas require acid soils and suffer iron deficiency in alkaline soils.
Alfalfa, apples, broccoli, and hydrangeas require an alkaline soil, whereas most plants do best if the soil pH is near 6.0 to 6.5. Some plants are able to tolerate a wide range of soil acidity, but typically plants show best health and vigor only in soil with the optimal pH.
THE ENDODERMIS AND SELECTIVE ABSORPTION OF SUBSTANCES
Elements in the soil solution, whether essential or not, can enter roots either by crossing a plasma membrane and entering the symplastic protoplasm phase of the plant or by diffusing along cell walls and intercellular spaces in the apoplastic phase. In the first method, the selective permeability of the plasma membrane and the presence or absence of molecular pumps control the entry of ions and molecules; certain substances can be excluded and others can be actively transported in. However, a substance can penetrate the root epidermis and cortex simply by moving through the water in cell walls and intercellular spaces. No metabolic control exists, and even harmful substances can enter. If all substances could enter the xylem transpiration stream, they would have access to all cells of the plant body.
The endodermis prevents uncontrolled, apoplastic diffusion in roots. The Casparian strips on all radial walls are impermeable to water and water-borne solutes; nothing can cross it simply by diffusion (Fig. 7). For a substance to penetrate beyond the root cortex, it must first enter the endodermal cell protoplasm by being accepted across the plasma membrane of a cell in the root epidermis, cortex, or endodermis. Its highly selective permeability allows the endodermis to control which elements enter the transpiration stream.

FIGURE 7:The endodermis in roots prevents uncontrolled diffusion of minerals into the xylem (X 50).
MYCORRHIZAE AND THE ABSORPTION OF PHOSPHORUS
The roots of most plants form a symbiotic association with soil fungi, and this relationship is called a mycorrhiza; the symbiosis permits plants to absorb phosphorus efficiently . In the most common type, vesicular/arbuscular mycorrhizae, some of the fungal filaments penetrate root cortex cells, then branch profusely, forming a small tree-shaped arbuscule inside the cell; other filaments swell into balloon-like vesicles . The fungus collects phosphorus from the soil and transports it into arbuscules, where it accumulates as granules. After the arbuscules fill with phosphorus, the granules gradually disappear as phosphorus is transported into the root cell protoplasm. Once the transfer is complete, the arbuscule collapses and the root cell returns to normal. This mycorrhizal symbiosis is essential to most plants; plants in sterilized soil grow poorly and show signs of phosphorus deficiency even if the soil contains adequate amounts of phosphorus (Fig. 8). In soils with very high levels of available phosphorus, mycorrhizae may be less important

FIGURE 8 :In the pot on the left, soil fungi were killed, and the plant suffers from phosphorus deficiency. In the two pots on the right, mycorrhizal fungi are present. (R .Rcmcadori/V'isuab Unlimited)



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|