


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 13-4-2017
Date: 19-7-2016
Date: 12-4-2017
|
Cells Build Supramolecular Structures
Macromolecules and their monomeric subunits differ greatly in size (Fig. 1–1). A molecule of alanine is less than 0.5 nm long. Hemoglobin, the oxygen-carrying protein of erythrocytes (red blood cells), consists of nearly 600 amino acid subunits in four long chains, folded into globular shapes and associated in a structure 5.5 nm in diameter. In turn, proteins are much smaller than ribosomes (about 20 nm in diameter), which are in turn much smaller than organelles such as mitochondria, typically 1,000 nm in diameter. It is a long jump from simple biomolecules to cellular structures that can be seen with the light microscope.
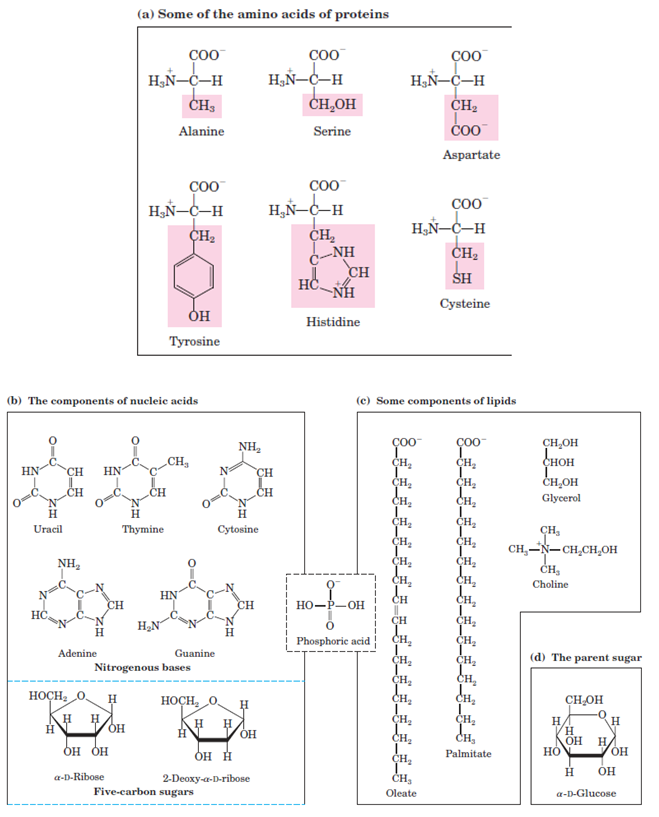
FIGURE 1–1 The organic compounds from which most cellular materials are constructed: the ABCs of biochemistry. Shown here are (a) six of the 20 amino acids from which all proteins are built (the side chains are shaded pink); (b) the five nitrogenous bases, two fivecarbon sugars, and phosphoric acid from which all nucleic acids are built; (c) five components of membrane lipids; and (d) D-glucose, the parent sugar from which most carbohydrates are derived. Note that phosphoric acid is a component of both nucleic acids and membrane lipids.
Figure 1–2 illustrates the structural hierarchy in cellular organization. The monomeric subunits in proteins, nucleic acids, and polysaccharides are joined by covalent bonds. In supramolecular complexes, however, macromolecules are held together by noncovalent interactions much weaker, individually, than covalent bonds.
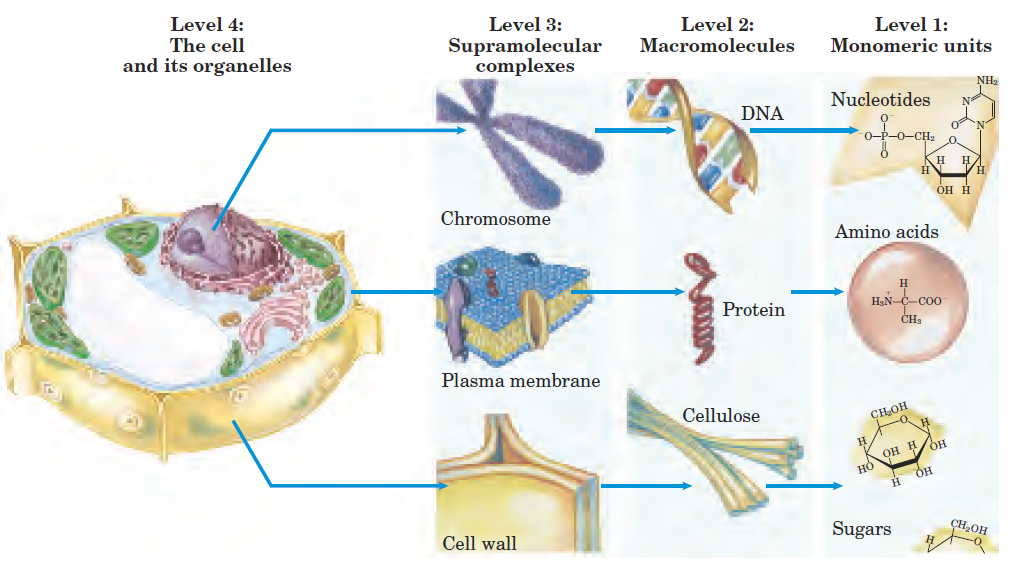
FIGURE 1–2 Structural hierarchy in the molecular organization of cells. In this plant cell, the nucleus is an organelle containing several types of supramolecular complexes, including chromosomes. Chromosomes consist of macromolecules of DNA and many different proteins. Each type of macromolecule is made up of simple subunits DNA of nucleotides (deoxyribonucleotides), for example.
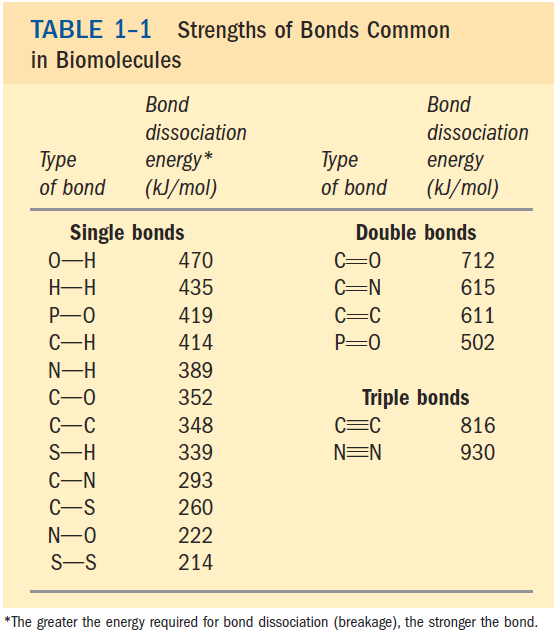
Among these noncovalent interactions are hydrogen bonds (between polar groups), ionic interactions (between charged groups), hydrophobic interactions (among nonpolar groups in aqueous solution), and van der Waals interactions all of which have energies substantially smaller than those of covalent bonds (Table 1–1). The large numbers of weak interactions between macromolecules in supramolecular complexes stabilize these assemblies, producing their unique structures.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|