

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Orientation of Electrophilic Additions: Markovnikov’s Rule
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
9th - p205
11-7-2016
2597
Orientation of Electrophilic Additions: Markovnikov’s Rule
Look carefully at the electrophilic addition reactions shown in the previous section. In each case, an unsymmetrically substituted alkene gives a single addition product rather than the mixture that might be expected. For example, 2-methylpropene might react with HCl to give both 2-chloro-2-methylpropane and 1-chloro-2-methylpropane, but it doesn’t. It gives only 2-chloro-2-methylpropane as the sole product. Similarly, it’s invariably the case in biological alkene addition reactions that only a single product is formed. We say that such reactions are regiospecific (ree-jee-oh-specific) when only one of two possible orientations of an addition occurs.
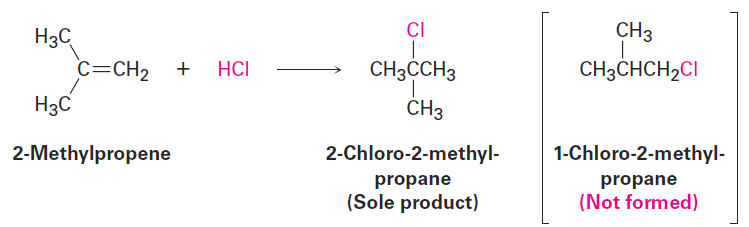
After looking at the results of many such reactions, the Russian chemist Vladimir Markovnikov proposed in 1869 what has become known as Markovnikov’s rule.
Markovnikov’s rule
In the addition of HX to an alkene, the H attaches to the carbon with fewer alkyl substituents and the X attaches to the carbon with more alkyl substituents.

When both double-bonded carbon atoms have the same degree of substitution, a mixture of addition products results.

Because carbocations are involved as intermediates in these electrophilic addition reactions, Markovnikov’s rule can be restated in the following way:
Markovnikov’s rule (restated)
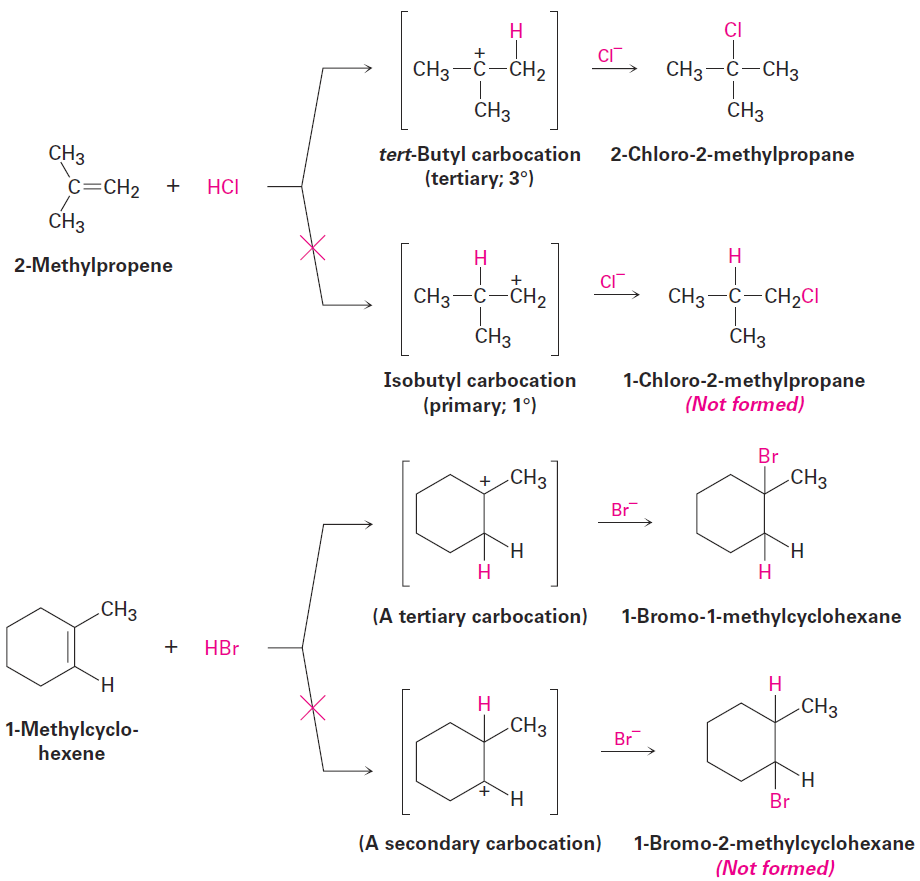
In the addition of HX to an alkene, the more highly substituted carbocation
is formed as the intermediate rather than the less highly substituted one. For example, addition of H+ to 2-methylpropene yields the intermediate tertiary carbocation rather than the alternative primary carbocation, and addition to 1-methylcyclohexene yields a tertiary cation rather than a secondary one. Why should this be?
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















