


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 12-12-2016
Date: 26-10-2020
Date: 5-2-2021
|
Gases and their Behaviour
Gases are the simplest of the different states of matter to understand. This is because the motion of the component molecules is essentially free apart from occasional collisions with other molecules.
Pressure. We have spoken of the pressure exerted by a gas on the sides of its container as being due to the continual pounding of these sides by the gas molecules. The magnitude of the pressure can be understood simply in terms of change of momentum. A molecule heading straight for the side of the container will have a certain momentum depending on its speed. When it strikes the side it will bounce back and its momentum will be reversed. This change in momentum implies that a force is exerted on the particle by the side and, in turn, because of Newton’s Third Law of Motion, an equal but opposite force is exerted on the side. This is the origin of gas pressure, which is simply defined as the total force exerted on unit area of the side by the gas molecules. Of course not all molecular collisions with the side are ‘head on’ and this has to be taken into account when working out exactly what the pressure is. It turns out that the pressure is roughly proportional to the average kinetic energy of the particles and, therefore, to the temperature of the gas. This is common experience; heat up gas in a container and eventually it will explode. It is also evident that the pressure must depend on how frequently the container sides are struck. It is possible, therefore, to increase the pressure in a gas simply by decreasing the volume of its container. For example, halve the volume occupied by a gas and its density will be doubled so twice as many molecules will be bombarding any side of the container. This means that the pressure has doubled. The same effect is experienced when a car tyre is inflated except that here the container size remains constant and more molecules of gas (air, in this case) are pumped into it, so increasing the density and, therefore, the pressure.
Condensation. Another feature of a gas is that if it is cooled then it condenses into a liquid; witness condensation of steam onto a cold window. This is understandable in qualitative terms. Cooling the gas reduces the speed of its component molecules. This means that when two molecules collide, if they are moving sufficiently slowly, the attractive intermolecular force is strong enough to hold them together. More and more collisions of this kind mean that a large number of molecules will be clinging together so the gas makes a transition into the liquid state.
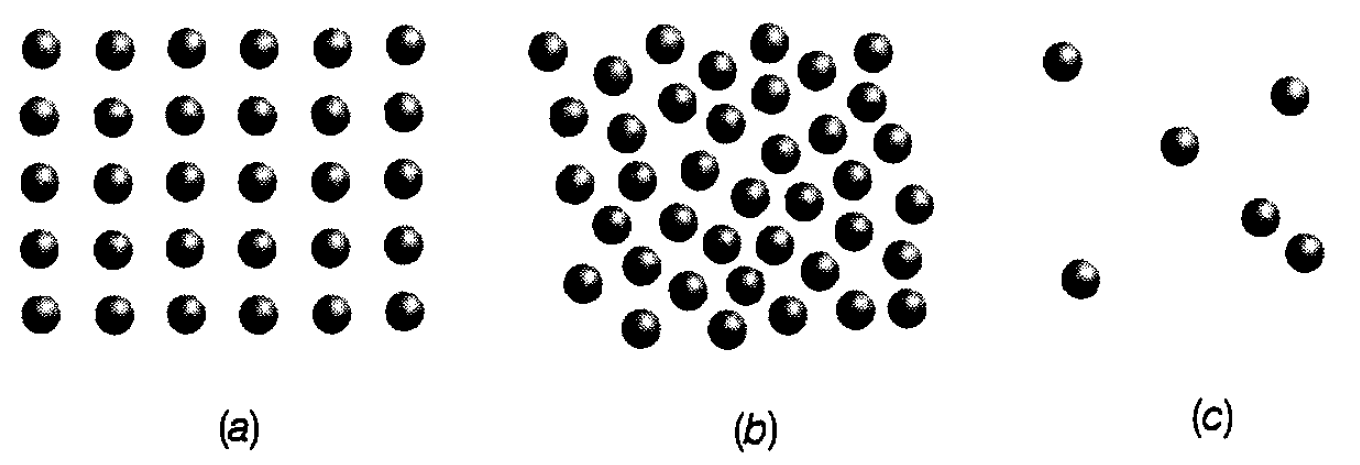
Figure 1.1: Instantaneous views of molecules in (a) a solid, (b) a liquid and (c) a gas.
It is a transition from figure 1.1(c) to figure 1.1(b). In terms of potential energy, it means that molecules sink towards the bottom of the valley illustrated in figure 1.l(b). If they are confined to the valley then, for energy to be conserved, their potential energy when they were approaching each other must have been handed over to the surroundings. In other words, heat is released. This heat must, in turn, be provided when a liquid is turned into a vapour and is, of course. The temperature at which a gas condenses into a liquid is obviously dependent on the strength of the intermolecular force-the weaker this force the lower the temperature. Helium, as has been mentioned earlier has a very weak interatomic force and temperatures only a few degrees above absolute zero are needed before it can be liquefied. It should also be stressed that temperatures at which condensation takes place are dependent on the gas pressure. For example, the higher the pressure the closer are the molecules and so the easier it is for them to hang together in liquid form.



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
العتبة العباسية المقدسة تطلق النسخة الحادية عشرة من مسابقة الجود العالمية للقصيدة العمودية
|
|
|