

 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 23-12-2015
Date: 29-12-2015
Date: 8-5-2021
|
Hematopoiesis
Hematopoiesis is a dynamic process in which blood cells of at least eight distinct lineages must be continually replaced from a population of pluripotent hematopoietic stem cells throughout the life span of the animal. The hematopoietic stem cell is a relatively rare cell, estimated to be approximately 0.001% of nucleated bone marrow cells. Stem cells are normally quiescent, in either the G0 or G1 phase of the cell cycle. Hematopoiesis involves the progressive restriction of the developmental potential of hematopoietic progenitors, with a concomitant increase in their proliferative capacity. Conversely, the self-renewal capacity of hematopoietic progenitor cells progressively decreases with the restriction of their developmental potential, with most mature cell types having short life spans and little or no proliferative capacity. In order to produce the correct numbers of mature blood cells, this balance between proliferation and differentiation of hematopoietic progenitors must be tightly controlled. Perturbation of this delicate balance can result in leukemogenesis or in anemias, immunodeficiencies, and other cellular deficiency disorders.
The existence of hematopoietic progenitor and stem cells can be inferred by the progeny they produce. Transplantation of lethally irradiated mice with cells from syngeneic donors has been the classical approach to demonstrate the presence of repopulating hematopoietic stem cells. The first 4 to 6 months after engraftment is characterized by clonal fluctuations in stem cell proliferation and differentiation, followed by the emergence of a stable hematopoietic system, dominated by a small number of totipotent clones. In addition, the extensive self-renewal capacity of these cells can be demonstrated by their ability to reconstitute recipient animals after serial transplantations. Competitive repopulation assays, in which two genetically distinct populations of bone marrow cells are transplanted together into the same recipient, can be used to determine the effects of a specific genetic alteration on the viability of repopulating hematopoietic stem cells.
The earliest restriction in developmental potential of hematopoietic stem cells involves the generation of putative lymphoid- and myeloid-restricted stem cells from the pluripotent myeloid-lymphoid stem cell. Myeloid-restricted stem cells can be detected by the classic spleen colony assay. In this assay, limiting numbers of bone marrow cells are injected into lethally irradiated recipient mice, giving rise to the formation of macroscopic hematopoietic colonies in the spleen within eight days, each derived from a single progenitor cell. These cells, termed CFU-S (colony-forming unit-spleen), are pluripotent and can give rise to all hematopoietic cells of the myeloid lineage. They possess only limited self-renewal capacity, as measured by their ability, on retransplantation, to give rise to secondary spleen colonies. In addition, CFU-S cannot readily confer hematopoietic repopulation and long-term survival of recipient mice. Additionally, progenitor cells that form spleen colonies at day 12 to 14 after transplantation are more potent in terms of repopulating ability than are day 8 CFU-S cells, suggesting that the spleen colony assay detects a range of cells that differ in their proliferative or repopulation potential.
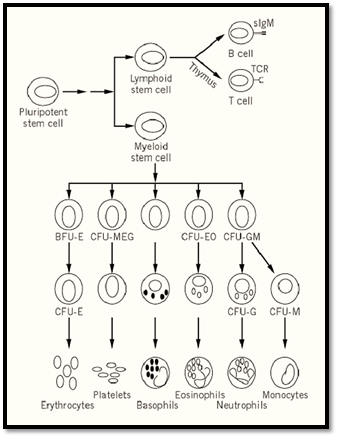
Figure 1. Schematic of adult hematopoiesis.
The presence of more restricted hematopoietic progenitor cells can be demonstrated by their ability to form colonies of differentiated blood cells in a semisolid medium in the presence of hematopoeitic growth factors. Multipotential hematopoietic cytokines, including interleukins IL-3 (multipotential CF), IL-4, and granulocyte/macrophage colony stimulating factor (GM-CSF), are capable of supporting the proliferation of multilineage progenitor cells, resulting in the formation of colonies containing multiple hematopoietic lineages. Lineage-specific growth factors, such as erythropoietin, granulocyte CSF (G-CSF), or macrophage CSF (M-CSF or CSF-1), enhance proliferation and differentiation of committed progenitor cells forming colonies of nucleated red blood cells, neutrophils, and macrophages, respectively. A final group of cytokines, including IL-6, IL-11, and Steel (stem cell factor, c-kit ligand), act synergistically with the multilineage and lineage-specific cytokines to enhance colony formation in culture by shortening the G0 period of the hematopoietic progenitors.
During mouse embryogenesis, the first visible sign of hematopoiesis appears in the extraembryonic mesoderm of the day 7.5 yolk sac in the form of blood islands. These blood islands contain primarily primitive erythrocytes, which are large, nucleated cells expressing embryonic hemoglobin, although adult globins are synthesized and accumulate in these cells at later stages of development. Cells of the monocyte-macrophage lineage also appear in the yolk sac. Although the differentiated hematopoietic cells found in the yolk sac are restricted to cells of the erythroid and macrophage lineages, progenitor cells found in the yolk sac at later stages of development can differentiate into various hematopoietic lineages in vitro and have been reported to be capable of forming CFU-S in vivo, demonstrating the multipotency of these cells. Furthermore, repopulation of recipient animals with yolk sac–derived hematopoietic cells has been reported, suggesting the presence of repopulating hematopoietic stem cells within the yolk sac environment.
Around day 10 of embryonic development, the primary site of hematopoiesis switches from the yolk sac to the fetal liver, the main site of fetal hematopoiesis. The fetal liver produces predominantly cells of the definitive erythroid lineage, expressing adult hemoglobins, as well as some myeloid and lymphoid cells. Various progenitor and stem cells, including CFU-S and pluripotent hematopoietic stem cells capable of long-term repopulation of recipient mice, are all present at a high frequency in the fetal liver. The fetal liver remains the primary erythropoietic organ until shortly before birth, when the major site of hematopoiesis shifts, once again, to the bone marrow. Within the intersinusoidal spaces of the medullary cavity of the bone, hematopoietic cells are found closely associated with the stroma. These cells directly influence hematopoiesis through cell–cell interactions and the production of cytokines, forming a microenvironment that supports blood cell production.
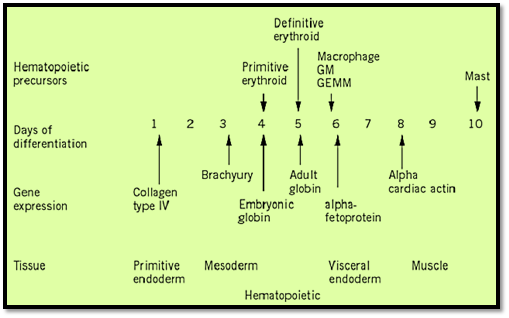
Figure 2. Time course of embryoid body development. Gene expression and presence of hematopoietic precursors are shown.
It has long been accepted that, during embryogenesis, the hematopoietic stem cells (HSCS) that originate in the yolk sac migrate to the fetal liver to initiate the second wave of hematopoiesis. However, recent findings of hematopoietic cells in the para-aortic splanchnopleure of mouse embryos prior to colonization of the fetal liver (1-3) suggest the possibility of another source of hematopoiesis in the embryo proper. It is unclear at this time whether the cells involved in primitive hematopoiesis in the yolk sac are precursors to the cells involved in definitive hematopoiesis or whether definitive hematopoiesis develops independently in the embryo proper. Transplantation studies using the adult mouse as a recipient demonstrated HSC activity in the aorta, gonads, and mesonephros (AGM) region of the embryo at day 10, followed by the fetal liver and yolk sac on day 11)4,3). These studies suggest that the first definitive HSCs arise in the AGM region and migrate to the fetal liver to establish definitive hematopoiesis. However, transplantation studies utilizing newborn or embryonic mice as recipients demonstrated the presence of HSCs contributing to definitive hematopoiesis in the yolk sac as early as day 9 (5), lending support to previous models. Although the origin of definitive hematopoiesis remains in debate, microenvironment is clearly a crucial element in the regulation of HSC potential.
The study of murine hematopoietic development, and the role of the microenvironment in this process, has been greatly facilitated by the establishment of in vitro model systems in which embryonic stem (ES) cells are differentiated to form cells of the hematopoietic system. Coculture of ES cells with primary bone marrow stroma or stromal cell lines results in the formation of a variety of hematopoietic cells including those of the erythroid, myeloid, B, and T cell lineages (6-8). Additionally, if the leukemia inhibitory factor is removed from the medium and the cells are grown in suspension, ES cells will form primitive embryonic structures termed embryoid bodies (EBs), which contain a vasculature and closely resemble the visceral yolk sac of the post-implantation embryo. These EBs contain many differentiated cell types, including those of the hematopoietic system, easily detectable by the appearance of visible blood islands containing red blood cells with hemoglobin.
ES-derived EBs express many genes that serve as markers of tissue restriction and lineage commitment during hematopoietic development (9-11), including the full complement of globin genes in the correct temporal order (12). EBs also contain precursors of the primitive erythroid, definitive erythroid, macrophage, neutrophil, and mast cell lineages, as demonstrated by colony-forming assays (10, 11, 13). Later in EB development, differentiated megakaryocytes, neutrophils, eosinophils, and mast cells also appear (14). Additionally, ES cell-derived EBs contain B and T cell precursors (15) and markers indicative of B and T lymphoid cells (16).
The in vitro differentiation of ES cells into cells of the hematopoietic system has been utilized to address the question of the origin of primitive versus definitive hematopoiesis. It remains unclear, however, whether primitive and definitive erythrocytes develop from common or distinct precursors in this system (17, 18). Recent evidence from Xenopus suggests the presence of bipotential primitive-definitive hematopoietic progenitors in the vertebrate embryo (19). However, the detectability of primitive erythrocytes in the yolk sac prior to the appearance of hematopoietic progenitor and stem cells in that tissue indicates that the traditional hierarchy of adult hematopoiesis may not apply in the context of primitive hematopoiesis.
The molecular events that regulate the development and maintenance of the hematopoietic system are beginning to come to light. A great deal of the work that forms the basis of this knowledge has been elucidated using the mouse as a model system. The combination of naturally occurring mutations and those generated by homologous recombination in ES cells has produced a battery of mutant ES and mouse lines, which have been instrumental in revealing the molecular biology of hematopoiesis. Furthermore, in vitro differentiation of homozygous mutant ES cells has provided additional information regarding the specific role of the targeted genes during hematopoietic development. Some of the genes that have been identified as essential for the development of hematopoietic stem cells during embryogenesis, or the functioning of these cells in the adult, are listed in Table 1.
Table 1. Hematopoietic Mutants

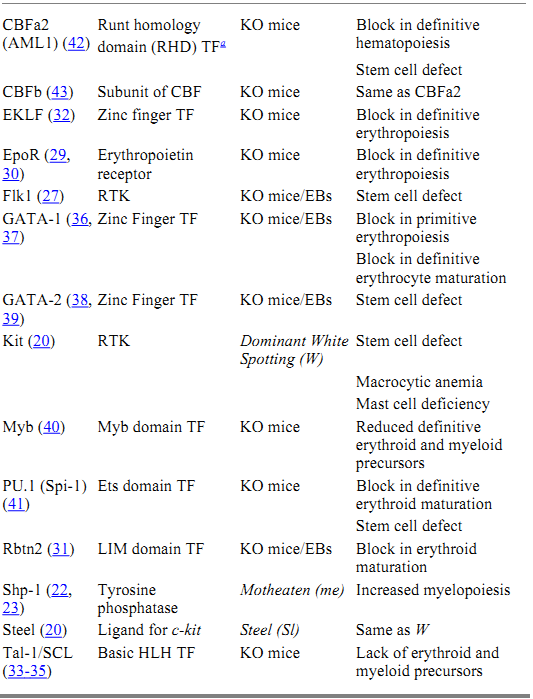
a Key: TF, transcription factor; RTK, receptor tyrosine kinase; KO, knockout; EBs, embryoid bodies.
The process of hematopoiesis is controlled by a number of hematopoietic growth factors, which bind to specific cell surface receptors and generate a complex series of intracellular signaling events. These signaling events culminate in changes in gene expression, which direct the cell toward proliferation or differentiation. The major strategy by which hematopoietic cells interpret extracellular signals is through the activation of receptors that either possess intrinsic tyrosine kinase activity or are coupled to cytoplasmic proteins with tyrosine kinase activity. Several of these receptors have been shown to play a role in the regulation of hematopoietic stem cells and to be crucial for the proper development of the hematopoietic system. Naturally occurring mutations in the receptor tyrosine kinase, c-kit (W), and its ligand, steel factor (Sl), have uncovered an important role for this signaling pathway in the regulation of hematopoietic stem cells (20). In addition to macrocytic anemia and mast cell deficiency, hematopoietic cells from W mice show defects in spleen colony formation and in long-term reconstitution of lethally irradiated recipient mice. In contrast, Sl mutant mice have a defective microenvironment that is unable to support the growth of normal stem cells. Genetic and biochemical analysis has revealed two other naturally occurring mutations that form part of the Kit-Steel signaling pathway (21). These mouse loci, motheaten (me) (22, 23) and micropthalmia ( mi) (24, 25), encode a protein tyrosine phosphatase (Shp-1) and a basic helix-loop-helix transcription factor (MITF), respectively.
Gene targeting strategies have also led to the identification of several hematopoietic receptors that are essential for normal hematopoietic development. One of these receptors is Flk1, a receptor tyrosine kinase that binds vascular endothelial growth factor (VEGF). Mice homozygous for a mutation in Flk1 lack both mature endothelial and hematopoietic cells (26). In addition, Flk1 -/- ES cells do not contribute to primitive or definitive hematopoiesis in vitro or in vivo (27), demonstrating a close association between the molecular and cellular regulation of vasculogenesis and hematopoiesis. Gene targeting of STK, a tyrosine kinase receptor of the Met family isolated from a hematopoietic stem cell cDNA library, has demonstrated a role for this receptor in the negative regulation of macrophage activation and nitric oxide production during a cell-mediated immune response (28.(
A second class of cell surface receptors that are important for hematopoietic differentiation contains the cytokine receptors, which signal through the Jak/Stat pathway. An example of this class of receptors, which effects the proliferation and survival of erythroid progenitors, is the erythropoietin (epo) receptor. Mice homozygous for mutations in epo or the epo receptor die at embryonic day 13 from a failure of definitive fetal liver erythropoiesis (29, 30). Committed BFU-E (burst-forming unit-erythroid) and CFU-E (colony-forming unit-erythroid) progenitors are present in these mice, suggesting that the epo signaling pathway is necessary for the proliferation and survival of CFU-E progenitors but not for erythroid lineage commitment.
The signaling events generated in response to the engagement of hematopoietic receptors by their respective ligands culminate in changes in gene expression, which direct the cell toward proliferation or differentiation. Genetic analysis in mice has also revealed several transcription factors that are essential for the development of hematopoietic precursors. Collectively, these transcription factors regulate the expression of hematopoietic-specific genes that are important for the differentiation of multiple blood cell lineages from the pluripotential hematopoietic stem cell. Some of these factors are specific for the development of the erythropoietic lineage (GATA-1, EKLF, Rbtn2), suggesting a role in erythroid commitment and/or maturation. Other factors play a more global role in regulating the proliferation versus differentiation of primitive hematopoietic progenitors (PU.1, GATA-2, Myb, SCL/Tal-1). Interestingly, these studies suggest that primitive and definitive hematopoiesis are controlled by somewhat distinct molecular mechanisms. Disruption of genes encoding Rbtn2 (31), SCL/Tal-1 (32-35), GATA-1 (36, 37), or GATA-2 (38, 39) block both primitive and definitive erythropoiesis, whereas mutations in c-myb (40), PU.1 (41), EKLF (32), and CBF a and b (42, 43) affect only definitive erythropoiesis.
The list of mouse mutations effecting the development of the hematopoietic system is growing rapidly. Furthermore, many mutations exist that result in mild alterations in the regulation of adult hematopoiesis rather than in a complete block in hematopoiesis. This finding suggests some redundancy in the pathways that ultimately culminate in proliferation and differentiation of hematopoietic cells. The generation of mice with mutations in multiple genes involved in the regulation of hematopoiesis will be instrumental in the characterization of hematopoietic signaling pathways, as well as in the identification of points at which these pathways intersect.
References
1. I. E. Godin et al. (1993) Nature 364, 67–70.
2. A. L. Medvinsky et al. (1993) Nature 364, 64–67.
3. A. M. Muller et al. (1994) Immunity 1, 291–301.
4. A. Medivinsky and E. Dzierzak (1996) Cell 86, 897–906.
5. M. C. Yoder et al. (1997) Immunity 7, 335–344.
6. T. Nakano, H. Kodama, and T. Honjo (1994) Science 265, 1098–1011.
7. R. Palacios, E. Golunski, and J. Samaridis (1995) Proc. Natl. Acad. Sci. USA 92, 7530–7534.
8. J. C. Gutierrez-Ramos and R. Palacios (1992) Proc. Natl. Acad. Sci. USA 89, 9171–9175.
9. T. McClanahan et al. (1993) Blood 81, 2903–2915.
10. G. Keller et al. (1993) Mol. Cell. Biol. 13, 473–486.
11. R. M. Schmitt, E. Bruyns, and H. R. Snodgrass (1991) Genes Dev. 5, 728–740.
12. M. H. Lindenbaum and F. Grosveld (1990) Genes Dev. 4, 2075–2085.
13. M. V. Wiles and G. Keller (1991) Development 111, 259–267.
14. U. Burkert, T. von Ruden, and E. F. Wagner (1991) New Biol. 3, 698–708.
15. A. J. Potocnik, P. J. Nielsen, and K. Eichmann (1994) EMBO J. 13, 5274–5283.
16. U. Chen, M. Kosco, and U. Staerz (1992) Proc. Natl. Acad. Sci. USA 89, 2541–2545.
17. M. Kennedy et al. (1997) Nature 386, 488–493.
18. T. Nakano, H. Kodama, and T. Honjo (1996) Science 272, 722–724.
19. J. B. Turpen et al. (1997) Immunity 7, 325–334.
20. A. Reith, and A. Bernstein (1991) Genome Analysis 3: Genes and Phenotypes (K. Davies and S. Tilghman, eds.) 105–133.
21. R. F. Paulson et al. (1996) Nature Genetics 13, 309–315.
22. L. D. Shultz et al. (1993) Cell 73, 1445–1454.
23. H. W. Tsui et al. (1993) Nature Genet 4, 124–129.
24. C. A. Hodgkinson et al. (1993) Cell 74, 395–404.
25. M. J. Hughes et al. (1993) J Biol Chem 28, 20687–20690.
26. F. Shalaby et al. (1995) Nature 376, 62–66.
27. F. Shalaby et al. (1997) Cell 89, 981–990.
28. P. H. Correll et al. (1997) Genes Function 1, 69–83.
29. H. Wu et al. (1995) Cell 83, 59–67.
30. C.-S. Liu et al. (1996) Genes Dev. 10, 154–164.
31. A. J. Warren et al. (1994) Cell 78, 45–57.
32. B. Nuez et al. (1995) Nature 375, 316–322.
33. C. Porcher et al. (1996) Cell 86, 47–57.
34. L. Robb et al. (1996) EMBO J. 15, 4123–4129.
35. R. A. Shivdasani, E. L. Mayer, and S. H. Orkin (1995) Nature 373, 432–434.
36. L. Pevny et al. (1991) Nature 349, 257–260.
37. M. J. Weiss, G. Keller, and S. H. Orkin (1994) Genes Dev 8, 1184–1197.
38. F.-Y. Tsai and S. H. Orkin (1997) Blood 89, 3636–3643.
39. F.-Y. Tsai et al. (1994) nature 371, 221–226.
40. M. L. Mucenski et al. (1991) Cell 65, 677–689.
41. E. W. Scott et al. (1994) Science 265, 1573–1577.
42. T. Okuda et al. (1996) Cell 84, 321–330.
43. Q. Wang et al. (1996) Cell 87, 697–708.



|
|
|
|
إجراء أول اختبار لدواء "ثوري" يتصدى لعدة أنواع من السرطان
|
|
|
|
|
|
|
دراسة تكشف "سببا غريبا" يعيق نمو الطيور
|
|
|
|
|
|
لأعضاء مدوّنة الكفيل السيد الصافي يؤكّد على تفعيل القصة والرواية المجسّدة للمبادئ الإسلامية والموجدة لحلول المشاكل المجتمعية
|
|
|
|
قسم الشؤون الفكرية يناقش سبل تعزيز التعاون المشترك مع المؤسّسات الأكاديمية في نيجيريا
|
|
|
|
ضمن برنامج عُرفاء المنصّة قسم التطوير يقيم ورشة في (فنّ الٕالقاء) لمنتسبي العتبة العباسية
|
|
|
|
وفد نيجيري يُشيد بمشروع المجمع العلمي لحفظ القرآن الكريم
|