


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 9-5-2021
Date: 10-12-2020
Date: 9-5-2016
|
Globins
1. Definition, structure, and properties
Globins are members of a family of oxygen-binding proteins, characterized by homologous amino acid sequences and a characteristic protein structure known as the “globin fold.” As illustrated in Figure 1, the globin fold is comprised of eight alpha-helical segments, designated A through H, which are connected by loop segments AB, BC, ¼ and GH. The nonhelical segments at the amino and carboxyl termini are designated as NA and HC, respectively. The globin fold is maintained even when the sequence identity between pairs of globins is as low as 16% (2). In most vertebrates, individual globin polypeptide chains consist of 141 to 153 amino acid residues. Another property of the globin fold is its ability to bind a heme group, protoporphyrin IX with a ferrous iron atom at its center. The heme group is inserted in a cleft in the protein structure between the E and F helices, known as the “heme pocket.” The iron atom is linked covalently to the imidazole N ; of the “proximal” histidine residue at position F8 (the eighth residue of the F helix). There are also many van der Waals interactions between the heme group and the amino acid side chains that line the heme pocket. Under physiological conditions, the iron atom is in the ferrous state, when it is capable of binding one molecule of O2 on the “distal” side of the heme group. The side chain of the distal His-E7 forms a hydrogen bond with one of the oxygen atoms, stabilizing the bound oxygen. In this state, the ferrous iron is hexacoordinated with one of the oxygen atoms, N ; of His-F8, and the four pyrrole nitrogen atoms of protoporphyrin IX. When oxygen is not bound, the sixth coordination site for oxygen is left vacant, and the iron atom is only pentacoordinated.
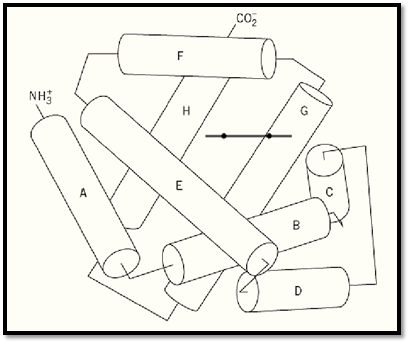
Figure 1. The globin fold. Cylinders represent the eight a-helices. The lines correspond to the interhelical regions are not exact and signify only the connectivity of the polypeptide chain. The bold short line indicates the side view of the heme group.
2. Distribution and Classification
Globins are found in organisms as hemoglobin (Hb) or myoglobin (Mb). Their common property is the reversible binding of oxygen. Mb is present in the muscles and stores oxygen, whereas Hb is present in the blood and transports oxygen from the lungs or gills to the peripheral tissues. Mb is usually a monomer, although sometimes a dimer, comprised of a single polypeptide chain and one heme group, whereas Hb is an oligomer or polymer composed two or more globin polypeptide chains, each with a heme group. Globin oxygen-binding proteins are even found in organisms that have no muscle or blood, including insects, plants, and single-celled organisms such as protozoa, yeast, and bacteria. These proteins are conventionally called hemoglobins, although some call them myoglobins because of their monomeric structures. Globin genes are widespread in modern plants, and plant hemoglobins are believed to have evolved from an evolutionary ancestor common to modern plants and animals (3).
3. Modular Structure and Evolution
The globin fold is composed of four structural units, called modules M1, M2, M3, and M4 (4) (Fig. 2). In most globins, modules M1 and M4 are encoded by two corresponding exons of the gene. In contrast, modules M2 and M3, which are the structural units that hold the heme group, are encoded by a single exon. In the Hb genes of leghemoglobin (from the legume root nodule) and nematode Hb, however, this single exon is divided into two exons by the insertion of one intron. It is believed that this intron was present initially in the ancestral globin gene but lost during the molecular evolution of the other globin genes (6). Consequently, the globin fold is considered to be encoded by four exons that correspond to the four structural modules. This is one of the best indications of protein evolution by exon shuffling.

Figure 2. Schematic representation of the three-dimensional structure of the human Hb-b chain and its constituent four structural modules. ( a) The structure of the b-chain; the heme group is bound in a pocket between helices E and F. Arrows indicate the positions of the modular joints that correspond to the positions of the introns in the b-globin gene. )b) The first module, at the N-terminus, encoded by Exon 1. (c) The joined second and third modules, encoded by Exon 2; the heme group is bound by this pair of modules. (d) The fourth module, encoded by Exon 3.
References
1. J.C. Kendrew, R.E. Dickerson, B.E. Strandberg, R.G. Hart, D.R. Davies, D.C. Phillips, and V.C. Shore (1960) Nature 185, 422–427.
2. A.M. Lesk and C. Chothia (1980) J. Mol. Biol. 136, 225–270.
3. J. Landsmann, E.S. Dennis, T.J.V. Higgins, C.A. Appleby, A.A. Kortt, and W.J. Peacock (1986( Nature 324, 166–168.
4. M. Go (1981) Nature 291, 90–92.
5. M. Go (1983) Shizen (in Japanese) 7, 26–35.
6. M. Go (1991) In Evolution of Life: Fossils, Molecules, and Culture (S. Osawa and T. Honjo, eds.), Springer-Verlag, Tokyo, pp. 109–122.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|