Most probable number (MPN) method APHA 2001 for total coliforms, thermotolerant coliforms and E. coli in foods
Method of the American Public Health Association (APHA), as described in the 4th Edition of the Compendium of Methods for the Microbiological Examination of Foods (Kornacki and Johnson, 2001). Included also recommendations from the Standard Methods for the Examination of Dairy Products (Davidson et al., 2004), specific to the examination of dairy products. In the Compendium is also applied to the examination of bottled water.
1 - Material required for analysis
Preparation of the sample and serial dilutions
• Diluent: 0.1% Peptone Water (PW) or Butterfield’s Phosphate Buffer Dilution tubes containing 9 ml 0.1% Peptone Water (PW) or Butterfield’s Phosphate Buffer
Total and thermotolerant coliforms count
• Lauryl Sulfate Tryptose (LST) Broth tubes (10 ml)
• Brilliant Green Bile (BGB) Broth 2% with Durham tubes
• E. coli (EC) Broth with Durham tubes
• Pure culture E. coli
• Pure culture E. aerogenes
• Laboratory incubator set to 35 ± 0.5°C
• Water bath set to 45.5 ± 0.2°C
• Water bath set to 44.5 ± 0.2°C
E. coli count (traditional method for foods) (optional)
• Levine’s Eosin Methylene Blue (L-EMB) Agar
• Plate Count Agar (PCA) slants
• Koser’s Citrate Broth or Simmons Citrate Agar slants
• Tryptone (Tryptophane) Broth
• MR-VP Broth
• Indole Kovacs Reagent
• Methyl Red Solution
• Voges-Proskauer (VP) Test Reagents (5% α-naphthol alcoholic solution, 40% potassium hydroxide aqueous solution, creatine phosphate crystals)
• Laboratory incubator set to 35 ± 0.5°C
2- Procedure
A general flowchart for the detection of total coliforms, thermotolerant coliforms and E. coli in foods by the MPN Method APHA 2001 is shown in Figure.1.
a) Preparation of the samples and inoculation: homogenize 25 g or 25 ml of sample with 225 ml of 0.1% Peptone Water (PW) or Butterfield’s Phosphate Buffer (10−1 dilution). If the food is acid check the pH of the 10−1 dilution and adjust to 6.8 if necessary (with sterile NaOH).
Prepare subsequent decimal dilutions of the sample. Select three appropriate dilutions and inoculate three 1 ml portions of each dilution onto three tubes with 10 ml of Lauryl Sulfate Tryptose Broth (LST).
Note a.1) Use 5-tube MPN series for analysis of shellfish and shellfish harvest waters.
If the expected count is low, inoculate three 10 ml aliquots of the 10−1 dilution onto three tubes with 10 ml of LST Broth double strength, three 1 ml aliquots of the 10−1 dilution onto three tubes with 10 ml of LST Broth single strength, and three 1 ml aliquots of the 10−2 dilution onto three tubes with 10 ml of LST Broth single strength.
For liquid samples with low count it is possible to start the series without dilution: 3 × 10 ml of sample without dilution onto 3 × 10 ml of LST Broth double strength, 3 × 1 ml of sample without dilution onto 3 × 10 ml of LST Broth single strength and 3 × 10 ml of 10−1 dilution onto 3 × 10 ml of LST Broth single strength. If the food is acid adjust the pH of the three double strength LST tubes to 6.8 after the inoculation (with sterile NaOH).
For liquid samples with low count it is also possible to apply a MPN single dilution test, inoculating 5 × 10 ml aliquots of the sample without dilution onto 5 × 10 ml of LST Broth double strength. If the food is acid adjust the pH of the five double strength LST tubes to 6.8 after the inoculation (with sterile NaOH).
b) Incubation for presumptive test: Incubate LST tubes at 35 ± 0.5°C/24 ± 2 h. Examine tubes and record reactions at 24 ± 2 h for gas (displacement of medium in fermentation vial or effervescence when tubes are gently agitated). Re-incubate gas-negative tubes for an additional 24 h and examine and record reactions again at 48 ± 2 h. Perform confirmed test on all presumptive positive (gas) tubes.
Note b.1) The accepted temperature variation in the Standard Methods for the Examination of Dairy Products (Davidson et al., 2004) is ± 1°C. In the Compendium of Methods for the Microbiological Examination of Foods (Kornacki and Johnson, 2001) is ± 0.5°C to harmonize with the Standard Methods for the Examination of Water and Wastewater (Hunt and Rice, 2005).

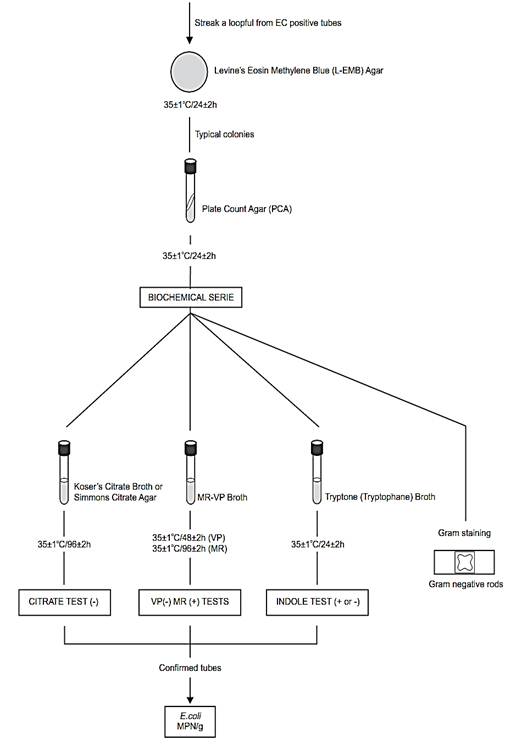
Figure .1 Scheme of analysis for the enumeration of total and thermotolerant coliforms and E. coli in foods using the MPN Method APHA 2001 (Kornacki and Johnson, 2001).
c) Confirmed test for total coliforms: From each gas-sing LST tube, transfer a loopful of suspension to a tube of Brilliant Green Bile Broth (BGB). Incubate BGB tubes at 35 ± 0.5°C/24 ± 2 h and examine for gas production, indicative of a positive result. Re-incubate gas-negative tubes for an additional 24 h and examine and record reactions again at 48 ± 2 h. Calculate most probable number (MPN) .
d) Confirmed test for thermotolerant (fecal) coliforms: From each gassing LST tube, transfer a loopful of suspension to a tube of E. coli Broth (EC).
Incubate EC tubes at 45.5 ± 0.2°C/24 ± 2 h for most foods and 44.5 ± 0.2°C/24 ± 2 h for waters and shellfish, preferably in a circulating water bath. If a variety of food types are to be examined, a single incubation temperature of 45.0 ± 0.2°C/24 ± 2 h should be suffice. Examine tubes for gas production, indicative of a positive result. Calculate the thermotolerant coliforms most probable number (MPN).
Note d.1) Place all the EC tubes in the water bath within 30 min after inoculation. Maintain a sufficient water depth in the water bath incubator to immerse the EC tubes to upper level of the medium. Incubate an EC tube of E. coli and an EC tube of E. aerogenes in the same water bath as positive and negative control.
Note d.2) If the analysis will continue (E. coli count completed test below), re-incubate negative EC tubes for an additional 24 h.
e) Completed test for E. coli in foods (optional): Procedure described by the Compendium (Kornacki and Johnson, 2001) and by the Standard Methods for the Examination of Dairy Products (Davidson et al., 2004).
From each gassing 48 h EC tube, streak (for isolation) a loopful to a Levine’s Eosin Methylene Blue Agar (L-EMB) plate. Incubate at 35 ± 1°C/24 ± 2 h and examine plates for suspicious E. coli colonies (dark centered and flat, with or without metallic sheen).
Transfer two suspicious colonies from each L-EMB plate to Plate Count Agar (PCA) slants, incubate at 35 ± 1°C/24 ± 2 h and use for further testing.
From PCA slants prepare a smear for Gram stain and inoculate cultures into the following broths, for confirmation by IMVC tests (indole, methyl red, Voges-Proskauer and citrate).
e.1) Citrate test: Lightly inoculate a tube of Koser’s Citrate Broth and incubate at 35 ± 1°C/96 ± 2 h. Development of distinct turbidity (growth) is positive reaction. Alternatively, Simmons Citrate Agar (slants) may be used. Inoculate by streaking slant and stabbing butt. Incubate at 35°C/96 h. Positive test is indicated by presence of growth, usually accompanied by color change from green to blue. Negative test is indicated by no growth or very little growth and no color change.
e.2) Indole test: Inoculate tube of Tryptone (Tryptophane) Broth and incubate at 35 ± 1°C/24 ± 2 h. Test for indole by adding 0.2–0.3 ml of Indole Kovacs Reagent. Appearance of distinct red color in upper layer is positive test and lack of red color is negative test).
e.3) Methyl red (MR) and Voges-Proskauer (VP) tests: Lightly inoculate tube of MR-VP Broth and incubate at 35 ± 1°C/48 ± 2. Perform Voges-Proskauer (VP) test at room temperature as follows: Transfer 1 ml 48 h culture to test tube and incubate remain-der of MR-VP broth an additional 48 h at 35 ± 1°C. Add Voges-Proskauer (VP) Test Reagents as follows: 0.6 ml α-naphthol, shake well, 0.2 ml of 40% KOH solution and shake. To intensify and speed reaction, add a few crystals of creatine. Read results after 4 h. Development of pink-to-ruby red color throughout medium is positive test and lack of red color is negative test. Perform methyl red test as follows: To 5 ml of 96 h MR-VP broth, add 5–6 drops of methyl red indicator.
Read results immediately. Most E. coli cultures give positive test, indicated by diffuse red color in medium. A distinct yellow color is negative test.
Consider as E. coli the cultures of Gram negative rods, indole negative or positive, MR positive, VP negative and citrate negative. Calculate most probable number (MPN) .
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Davidson, P.M., Roth, L.A. & Gambrel-Lenarz, S.A. (2004) Coliform and other indicator bacteria. In: Wehr, H.M. & Frank, J.F (eds). Standard Methods for the Examination of Dairy Products. 17th edition. Washington, American Public Health Association. Chapter 7, pp. 187–226.
Kornacki, J.L. & Johnson, J.L. (2001) Enterobacteriaceae, coliforms, and Escherichia coli as quality and safety indicators. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbio-logical Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 8, pp. 69–82.
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


