


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 25-4-2016
Date: 3-6-2021
Date: 16-4-2021
|
Bisubstrate Analogue
Bisubstrate analogues were developed originally for mechanistic studies on enzymes that catalyze reactions with two substrates or products. However, they have also proved to be of value in studies on enzymes by X-ray crystallography. Bisubstrate analogues are characterized by the fact that they embody in a single molecule the structural features of each of the two substrates (or products). Hence, it was expected that they would bind simultaneously at the binding sites for the two substrates, and therefore more tightly than the individual substrate molecules, and act as potent enzyme inhibitors. They have also been referred to as transition state analogues, but such a classification seems inappropriate.
Two early, and now classical, examples of bisubstrate enzyme inhibitors are P1, P5-di-(adenosine-5′)pentaphosphate (AP5A) and N-phosphonoacetyl-L-aspartate (PALA), whose structures are given in Figure 1. AP5A was developed as an inhibitor of adenylate kinase, which is a monomeric enzyme that catalyzes the reaction:
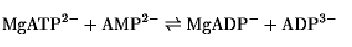
The reaction conforms to a rapid equilibrium, random kinetic mechanism, which implies that the enzyme possesses two distinct nucleotide-binding sites within its active site. One is for either MgATP2– or MgADP– and the other is for ADP3– or AMP2–. Irrespective of whether they are substrates or products, the moieties bound by the enzyme include two adenosine moieties and four phosphate moieties, whereas AP5A differs only in having five phosphate groups linked covalently. AP5A is a potent inhibitor of the adenylate kinase reaction, with the apparent inhibition constant Ki value being in the nanomolar region; the inhibition is competitive with respect to both MgATP and AMP, as would be expected (1, 2). Binding studies indicate that the stoichiometry of binding is 1:1 and the dissociation constant is 15 nM (3). The binding affinity is reduced seven-fold in the absence of Mg2+. It is of interest that the binding to adenylate kinase of AP4A, which is the equivalent of covalently linking ATP to AMP, or ADP to ADP, is almost 3,000-fold weaker. An increase in the number of phosphoryl groups to six also reduces the binding affinity by 400-fold. The crystal structures of three adenylate kinases and enzyme–AP5A complexes were solved well ahead of the spatial assignment of the substrate binding sites. A review of the problems associated with the determination of the structural relationship between the two nucleotide binding sites of adenylate kinase has been presented (4).
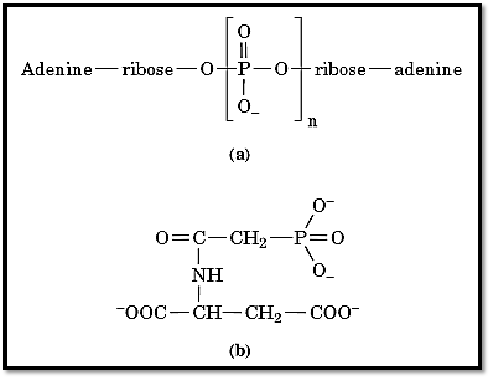
Figure 1. Examples of bisubstrate analogues. (a), P1, Pn-di(adenosine-5′) n-phosphate, where n = 4 (AP4A), 5 (AP5A), or 6 (AP6A). (b), N-phosphonoacetyl-L-aspartate (PALA).
AP5A also acts as a strong inhibitor of ATP:NMP phosphotransferase from Dictyostelium discoideum, which utilizes either UMP or CMP as the acceptor of a phosphoryl group from ATP (5). Binding studies indicate that the dissociation constant of the enzyme-AP5A complex is 160 µM. However, the corresponding bisubstrate analogue with uridine replacing one adenine nucleotide ) UP5A) is a more potent inhibitor and binds with a dissociation constant of 3 nM. The enzyme has been co-crystallized with UP5A.
PALA has been used extensively for kinetic and structural studies on aspartate transcarbamoylase (ATCase), which catalyzes the reaction:
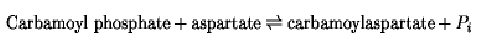
PALA is considered an analogue of the two substrates linked covalently. The intact ATCase enzyme consists of both catalytic and regulatory units, does not show Michaelis–Menten kinetics, and is subject to allosteric control by nucleoside triphosphates. However, it is possible to prepare an active trimer of just the catalytic subunits that shows Michaelis–Menten kinetics and is not subject to allosteric control. This form of enzyme has been very useful for elucidating fundamental information about the catalytic sites and mechanism of action of ATCase. Investigations with the catalytic trimer have shown that one molecule of PALA binds to each subunit of the trimer (6) and the inhibition is linear competitive with respect to carbamoyl phosphate and linear noncompetitive relative to aspartate (7). Additional kinetic data have indicated that the kinetic mechanism for the aspartate transcarbamoylase reaction is essentially ordered, with carbamoyl phosphate being the first substrate to add; this explains the inhibition pattern. The pH-independent Ki value for the enzyme-PALA complex is 7.2 nM, which is three orders of magnitude lower than the dissociation constant for the corresponding enzyme-carbamoyl phosphate complex (7).
PALA has also played an important role in the demonstration that the co-operativity observed with intact ATCase can be explained in terms of a two-state allosteric concerted model (8). Thus, the binding of up to three molecules of PALA to the intact enzyme, which has six active sites, leads to activation, even though PALA is occupying active sites, as a result of displacing the equilibrium between the T (inactive) and R (active) forms of the enzyme toward R. Only with greater occupancy is PALA inhibitory. Structural studies support the idea that PALA is a true bisubstrate analogue of the substrates for the enzyme (9).
References
1.G. E. Lienhard and I. I. Secemski (1973) J. Biol. Chem. 248, 1121–1123.
2.P. Feldhaus, T. Frohlich, R. S. Goody, M. Isakov, and R. H. Schirmer (1975) Eur. J. Biochem. 57,197-204.
3.J. Reinstein, I. R. Vetter, I. Schlichting, P. Rosch, A. Wittinghofer, and R. S. Goody (1990(Biochemistry 29, 7440–7450.
4.M.-D. Tsai and H. Yan (1991) Biochemistry 30, 6806–6818.
5.L. Wiesmuller, K. Scheffzek, W. Kliche, R. S. Goody, A. Wittinghofer, and J. Reinstein (1995( FEBS Lett. 363, 22–24.
6.G. R. Jacobson and G. R. Stark (1973) The Enzymes 9, 225–308.
7.J. L. Turnbull, G. L. Waldrop, and H. K. Schachman (1992) Biochemistry 31, 6562–6569.
8.L. E. Parmentier, M. H. O''Leary, H. K. Schachman, and W. W. Cleland (1992) Biochemistry 31, 6598-6602.
9. W. N. Lipscomb (1994) Adv. Enzymol. 68, 67–151. References



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
مشاتل الكفيل تزيّن مجمّع أبي الفضل العبّاس (عليه السلام) بالورد استعدادًا لحفل التخرج المركزي
|
|
|