


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 19-12-2015
Date: 10-12-2015
Date: 15-11-2020
|
Aspartic Acid (Asp, D(
The amino acid aspartic acid is incorporated into the nascent polypeptide chain during protein biosynthesis in response to only two codons—GAU and GAG—and represents approximately 5.3% of the residues of the proteins that have been characterized. The aspartyl residue incorporated has a mass of 115.09 Da, a van der Waals volume of 91 Å3, and an accessible surface area of 151 Å2. Asp residues are frequently changed during divergent evolution; they are interchanged in homologous proteins most frequently with asparagine and glutamic acid residues.
The side chain of Asp residues is dominated by its carboxyl group:
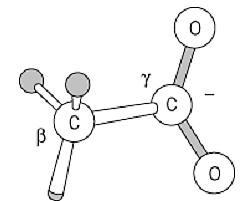
This carboxyl group is normally no more reactive than are those of corresponding organic molecules, such as acetic acid. Its intrinsic pKa value is close to 3.9, so Asp residues are ionized and very polar under physiological conditions; consequently, very few Asp residues are buried in folded protein structures, and nearly all have at least the carboxyl group on the surface. The pKa can be shifted in folded proteins, however, and either the ionized or nonionized form can be used in the protein's function. For example, carboxyl proteinases have one active-site carboxyl group function in the ionized form, and another when nonionized. Asp carboxyl groups have a weak intrinsic affinity for Ca2+ ions, and they are used in many calcium-binding proteins.
Asp residues differ from Glu only in having one methylene group, rather than two, so it might be thought that they would be very similar chemically and functionally in proteins, but this is not so. The slight difference in length of the side chains causes them to have different tendencies in their chemical interactions with the peptide backbone, so they have markedly different effects on the conformation and chemical reactivity of the peptide backbone. For example, Asp residues favor the alpha-helical conformation much less than Glu residues. In folded protein structures, Asp residues occur most frequently in reverse turns, whereas Glu residues are most frequently found in a-helices. The polypeptide chain can be cleaved relatively easily at Asp residues, because the side-chain carboxyl group participates in the reaction. -Asp- Pro- peptide bonds are especially labile in acid, because the carboxyl group interacts with the unique tertiary N atom of the Pro residue.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|