


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 4-5-2021
Date: 2-5-2016
Date: 22-4-2021
|
Antifreeze Proteins
Antifreeze proteins (AFPs) adsorb specifically to the surface of ice. By doing so, they inhibit its growth and cause a nonequilibrium depression of the freezing point below the colligative melting/freezing point (1). This depression, which is referred to as thermal hysteresis (°C), helps organisms to avoid or resist freezing when they encounter ice at temperatures below their colligative freezing points. For example, in polar and cold temperate marine environments bony fishes (teleosts) can encounter icy seawater at its freezing point of ≈ –1.9°C, which is over 1°C colder than their colligative freezing point of ≈ –0.7°C. Those fishes that have adapted to this environment produce high concentrations of AFP in their blood (10–25 mg/mL) that can depress their freezing point by the crucial 1°C needed for survival. For practical reasons, AFPs (sometimes known as thermal hysteresis proteins) have been best characterized in fish, but have also been found in insects, plants, and bacteria that encounter subzero conditions. Not all of these organisms resist freezing. In those that do freeze, AFPs may enhance their freeze tolerance by keeping ice crystals from growing (recrystallizing) in the frozen state (2). Recrystallization of ice occurs as the melting temperature is approached and ice becomes more dynamic. It is effectively inhibited by very low AFP concentrations (µg/mL). Although there are reports of AFPs neutralizing ice nucleators (3) and of having protective effects on cells above 0°C (4), the two well-established functions of macromolecular antifreezes, thermal hysteresis and inhibition of ice recrystallization, are related activities that depend on AFP interaction with ice by an absorption-inhibition mechanism.
1. Inhibition of Ice Growth
In ice, each water molecule makes four tetrahedrally oriented hydrogen bonds to neighboring molecules, and the resulting ice lattice has an underlying hexagonal symmetry. When an aqueous solution is cooled in the presence of an ice crystal, water adds preferably to the atomically rough prism surfaces of the crystal much faster than to the two smoother basal planes. At slight undercooling below the freezing point, the prism surfaces are curved, and the ice grows radially as a disk (Fig. 1). Most solutes are swept aside by the expanding ice front. However, AFPs with a specific affinity for an ice surface plane become adsorbed to that surface and cause growth inhibition that further defines the surface plane and typically shapes the ice crystal into a hexagonal bipyramid. The basis for growth inhibition at the surface of ice is that water is forced to add to the lattice between the bound AFP (1, 5). These submicroscopic ice fronts are constrained to grow with a surface curvature that makes it thermodynamically unfavorable for water to join the lattice, which in turn leads to a local depression of the freezing point below that of the bulk solvent. Rather than completely covering the surface, AFPs have been likened to buttons on a mattress (Fig. 1d).
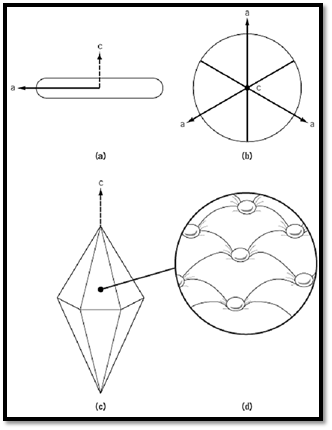
Figure 1. i) In the absence of AFP, a single ice crystal from the melt will grow fastest along the crystallographic a axes to form a disk with curved prism surfaces. ii) View of disk perpendicular to i) with c axis perpendicular to the page. iii( AFP adsorption to any aspect of the prism surface will force the crystal to become a hexagonal bipyramid. iv) The inset shows a section of the surface illustrating the submicroscopic ice front curvature between bound AFPs.
The relationship between thermal hysteresis and AFP concentration is nonlinear. Thermal hysteresis increases hyperbolically with increasing AFP concentration and approaches a plateau value of between 1 and 1.5°C for fish AFPs and 5 to 6°C for insect AFPs. If the temperature falls below the nonequilibrium freezing point at moderate to high AFP concentrations, the ice crystal will grow uncontrollably. At low AFP concentrations and moderate undercooling, the ice front will overgrow bound AFPs. This phenomenon has been used to deduce the ice surface to which AFPs bind by growing ice in a dilute AFP solution and then allowing it to sublime (ice etching) (5). The position of the AFP residue etch on the ice indicates its binding plane and, in some cases, its orientation of binding on that plane.
2. Adsorption of AFPs to Ice
One of the most intriguing features of AFPs is their structural diversity. In fish, there are at least five unrelated types, which are also different from insect AFPs (Fig. 2). It is likely that additional types will be discovered. They are so different that it has not been possible to identify a shared sequence or structural motif that could explain their common ice-binding activity. An added complication is that they bind to different ice surface planes (6). Early models for binding of AFPs to ice relied on a hydrogen-bonding match to the ice lattice (5, 7). However, it has been argued that hydrogen bonding of AFPs to liquid water is energetically favored over binding to ice (8). Also, correct identification of he ice-binding sites now shows them to be more hydrophobic than originally thought (9). One structural feature that AFPs have in common is an extensive region of intimate surface complementarity between the AFP and ice. Thus the energetic contributions to binding could include van der Waals interactions over the AFP–ice interface, some hydrogen bonds, and an entropic component from not having to solvate the AFP and ice surfaces in contact (10)
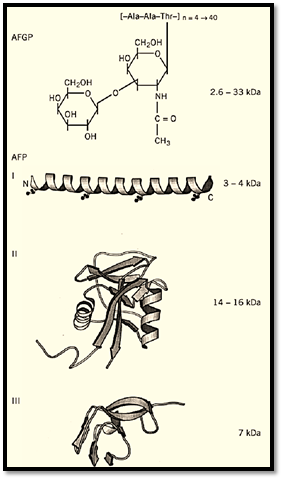
Figure 2. AFP structures. The 3D structures of Type I AFP from winter flounder (1WFA); Type II AFP from sea raven (2AFP); Type III AFP from ocean pout (1MSI); beetle AFP (1EZG); and moth AFP were solved by X-ray crystallography or nuclear magnetic resonance. The approximate masses of the proteins are indicated in kDa.
3. Evolution of AFPs
Fish AFPs have been classified into five different types. Antifreeze glycoproteins (AFGPs) are polymers of the tripeptide repeat Ala-Ala-Thr with a disaccharide (b-D-galactopyranosyl-(1→3)-2-acetamido-2-deoxy-a-D-galactopyranose) attached to the Thr. Type I AFP is an alanine-rich single a-helix. Type II AFP is a globular protein with sequence homology and structural similarity to the carbohydrate recognition domain of calcium-dependent lectins. Type III AFP is a smaller protein with a unique ß-sandwich fold that is homologous to a portion of sialic acid synthase. The most recently discovered (Type IV) AFP has homology to apolipoprotein E and is thought to be a helix-bundle protein.
It is clear that the different AFP types have distinct origins, which suggests that they have been independently co-opted as antifreezes relatively recently in the 175-million-year radiation of the bony fishes (11). Indeed, there are now several examples of very closely related fishes producing different AFP types and at least one clear example of distantly related fishes producing the same type through convergent evolution (12). This pattern of recent evolution is reinforced by the observation that AFP gene families show evidence of extensive amplification, often with the AFP genes organized as tandem repeats and with substantial variation in gene dosage (13). The diversity of AFP types is also reflected in their expression and processing. AFP genes are either constitutively expressed or are regulated by a variety of cues, including temperature, photoperiod, and hormonal cycles (14). Fish AFPs are typically produced in the liver as pre-proteins for secretion into the serum with or without a pro-sequence. The small antifreeze glycoproteins are produced from a large polyprotein by both proteolysis and glycosylation (12). In winter flounder, the external tissues, skin, scales, fins, and gills produce isoforms representing a subclass of Type I AFP that appears to lack a signal sequence (15).
Not unexpectedly, plant and insect AFPs are also different. AFPs from winter rye appear to be homologs of three types of extracellular plant pathogenesis-related sequences, namely endochitinases, endoglucanases, and thaumatin-like proteins (16), and carrot AFP is related to polygalacturonase inhibitor (17). These similarities reinforce the links between AFPs and sugar/polysaccharide-binding proteins. Plant AFPs appear to be more active in ice recrystallization inhibition than thermal hysteresis, whereas insect AFPs show considerably more thermal hysteresis activity than fish AFPs. AFPs from moths and beetles have distinct origins, and yet they both form ß-helices with similar threonine-rich ice-binding sites (18, 19). In some terrestrial insects that can resist freezing at temperatures down to –30°C, AFPs may be part of a suite of cryoprotective mechanisms that include elevated polyol levels and the elimination of ice nucleators that would interfere with supercooling.
4. Conclusion
Although adsorption-inhibition is the generally accepted mechanism of AFP action, the energetics of tight binding to ice are not yet fully understood. Other areas of current research include the basis for the hyperactivity of insect AFPs, activities of AFPs other than thermal hysteresis, and applications for AFPs in conferring freeze tolerance/resistance to unprotected organisms.
References
1. P. W. Wilson (1993) Cryo-Letters 14, 31–36.
2. C. A. Knight, A. L. DeVries, and L. D. Oolman (1984) Nature 308, 295–296.
3. A. Parody-Morreale, K. P. Murphy, E. DiCera, R. Fall, A. L. DeVries and S. J. Gill (1988( Nature 333, 782–783.
4. B. Rubinsky, A. Arav, and G. L. Fletcher (1991) Biochem. Biophys. Res. Commun. 180, 566–571 .
5. C. A. Knight, C. C. Cheng and A. L. DeVries (1991) Biophys. J. 59, 409–418.
6. C. C. Cheng and A. L. DeVries (1991) Life Under Extreme Conditions (G. di Prisco, ed.) , Springer-Verlag, Berlin, pp. 1–14.
7. D. Wen and R. A. Laursen (1992) Biophys. J. 63, 1659–1662.
8. A. D. J. Haymet, L. G. Ward, and M. M. Harding (1999) J. Am. Chem. Soc. 121, 941–948.
9. J. Baardsnes, M. E. Houston, Jr., H. Chao, R. S. Hodges, C. M. Kay, and P. L. Davies (1999(FEBS Letters 463, 87–91.
10. F. D. Sönnichsen, C. I. DeLuca, P. L. Davies and B. D. Sykes (1996) Structure 4, 1325–1337.
11. G. K. Scott, G. L. Fletcher, and P. L. Davies (1986) Can. J. Fisheries Aquatic Sci. 43, 1028–1034 .
12. L. Chen, A. L. DeVries, and C. H. C. Cheng (1997) Proc. Natl. Acad. Sci. USA 94, 3817–3822.
13. C. L. Hew, N. C. Wang, S. Joshi, G. L. Fletcher, G. K. Scott, P. H. Hayes, B. Buettner, and P. L. Davies (1988) J. Biol. Chem. 263, 12049–12055.
14. P. L. Davies, C. L. Hew, and G. L. Fletcher (1988) Can. J. Zool. 66, 2611–2617.
15. Z. Gong, K. V. Ewart, Z. Hu, G. L. Fletcher, and C. L. Hew (1996) J. Biol. Chem. 271, 4106–4112 .
16. W. C. Hon, M. Griffiths, A. Mlynraz, Y. C. Kwok, and D. S. C. Yang (1995) Plant Physiol. 109 , 879–989
17. D. Worrall, L. Elias, D. Ashford, M. Smallwood, C. Sidebottom, P. Lillford, J. Telford, C. Holt, and D. Bowles (1998) Science 282, 115–117.
18. Y.-C. Liou, A. Tocilj, P. L. Davies, and Z. Jia (2000) Nature 406, 322–324.
19. S. P. Graether, M. J. Kuiper, S. M. Gagne, V. K. Walker, Z. Jia, B. D. Sykes, and P. L. Davies (2000) Nature 406, 325–328.



|
|
|
|
حمية العقل.. نظام صحي لإطالة شباب دماغك
|
|
|
|
|
|
|
إيرباص تكشف عن نموذج تجريبي من نصف طائرة ونصف هليكوبتر
|
|
|
|
|
|
بمشاركة 60 ألف طالب.. المجمع العلمي يستعدّ لإطلاق مشروع الدورات القرآنية الصيفية
|
|
|
|
صدور العدد الـ 33 من مجلة (الاستغراب) المحكمة
|
|
|
|
المجمع العلمي ينظّم ورشة تطويرية لأساتذة الدورات القرآنية في كربلاء
|
|
|
|
شعبة التوجيه الديني النسوي تختتم دورتها الثانية لتعليم مناسك الحجّ
|