


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 2-12-2015
Date: 3-2-2016
Date: 28-2-2016
|
Fraction of Surface Atoms
Consider a homogeneous solid material of compact shape (let us say spherical) and macroscopic dimensions (let us say millimetric). Most of its properties will be related to its chemical composition and crystal structure. This is what is traditionally studied in the physics and chemistry of solids. For an object of this size, the surface atoms comprise a negligible proportion of the total number of atoms and will therefore play a negligible role in the bulk properties of the material. Note, however, that surface atoms will nevertheless play a predominant role in properties involving exchanges at the interface between the object and the surrounding medium. This is the case, for example, when we consider chemical reactivity (and catalysis) and crystal growth, which are discussed later in the book.
It can be seen from Fig. 1.1 that, when the size of the object is reduced to the nanometric range, i.e., < 10 nm, the proportion of surface atoms is no longer negligible. Hence, at 5nm (around 8,000 atoms), this proportion is about 20%, whilst at 2nm (around 500 atoms), it stands at 50%.
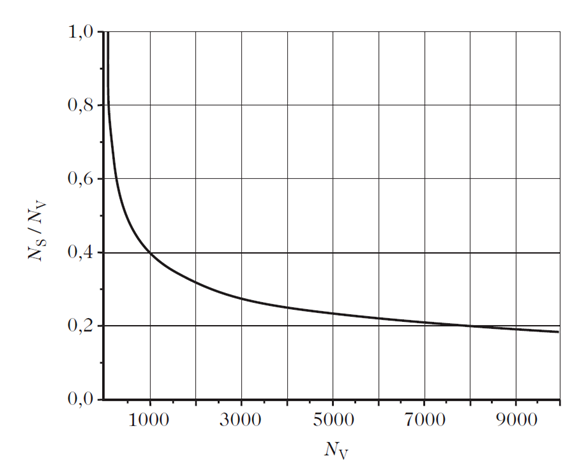
1. Proportion of surface atoms for a spherical particles comprising Nv atoms with Ns at the surface
This proportion can be estimated for the transition metals by the relationwhere R is the radius in nm.

This empirical law gives a proportion of surface atoms of 100% for a size of 1 nm.. We shall see that the fact that a large fraction of the atoms are located at the surface of the object will modify its properties. To tackle this question, we shall need to review certain physical quantities associated with surfaces, namely the specific surface energy and the surface stress.



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
مشاتل الكفيل تزيّن مجمّع أبي الفضل العبّاس (عليه السلام) بالورد استعدادًا لحفل التخرج المركزي
|
|
|