


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 21-2-2016
Date: 2025-02-09
Date: 21-2-2016
|
In MS, lesions comprising huge infiltrates of immune cells be present in the central nervous system (CNS) and are the disease hallmarks. These cells display the remarkable capacity to collapse the blood–brain barrier (BBB), get in into the parenchyma of brain, and begin the loss of neuronprotecting oligodendrocytes with later dissection of neuronal axons and eventually stimulating neuronal cell death (Al-Obaidi et al., 2021).
The disease is thought to start with the self-reactive effectors T cells activation in the periphery, which break the BBB of CNS. Once in the CNS, by local APCs, they are reactivated and recruit extra macrophages and T cells to institute the inflammatory lesion. T cells have been found to be located in these CNS permeations at early time points in the illness (Wingerchuk & Carter,2014).
Aberrant activity of these effector cell types might, in part, reflect a deficiency in the normal function of CD4+ regulatory T cells (T regs), (Baecher et al.,2018).
The importance of the balance between Th1 and Th17 cells and Tregs in CNS inflammation has been reinforced through multiple studies in animal models and in patients (Mexhitaj et al.,2019;Reich et al.,2018 and Steinman,2014) Notably, all immune cell subsets implicated in multiple sclerosis also serve normal functions, and these subsets can be modulated by a range of environmental exposures, including infections, the gut microbiome, vitamin D, adiposity, and smoking In addition to the cytokines and transcription factors traditionally used to define T-cell subsets (e.g., IFNγ and Tbet for Th1, IL-17 and RORγt for Th17), their expression of particular molecules, including adhesion molecules and chemokine receptors, has been considered relevant to several important steps in their contribution to the pathogenesis of relapsing multiple sclerosis. Such steps include immune activation, trafficking across the blood– CNS barrier (via adhesion, chemoattraction, and infiltration mechanisms), as well as presumed reactivation within the CNS with consequent damage to CNS elements (Bar-Or & Li, 2021).
The CD4+ T cells with cytotoxic potential might also contribute to the pathogenesis of multiple sclerosis, as either injury-promoting cells, or potentially in a beneficial way through regulated destruction of other proinflammatory cells (Peeters et al.,2017).
The significant role of CD4+ve T cells was also augmented by genetic studies recognizing status of HLA-DRB allele as the strongest genetic risk factor for the MS development (Consortium et al., 2019).
However, the population of CD4+ve T cells found in lesions of MS is somewhat small. Actually, these cells are greatly outnumbered by MHC-I limited CD8+ve T cells (Rodriguez,2007). Moreover, in MS, clonal expansion of T cells predominantly occurs in CD8+ve lineages (Babbe et al.,2000). significantly, neural structures that are target in MS, including glia and neurons, not express MHC-II but express MHC-I (Hoftberger et al.,2004). Developments have as well specified that Th17 proinflammatory T cells are plentiful in patients with MS and their existence appears correlated with activity of relapse positively. This increases the probability of targeting a novel and separate population of T-cell for curative intervention (Dwyer,2020).
The first step in activated cell immunity is to activate dendritic cells (DC) by toll-like receptors, which may identify certain microbial products. Dendritic cells get activated upon ligation, and these cells begin producing type I IFNs. T cells (signal-1) connect with DC in the secondary lymphoid organ via the HLA class II protein, which uniquely recognizes the T-cell receptor (TCR) on the surface of activated CD4+ T cells (signal-1) (Figure 1). However, activation of T cells also requires additional signals. Interaction with HLA and TCR induces activation of CD40 ligand (CD154) on the surface of T cells, which binds to its receptor CD40 on the DC. This interaction induces upregulation of CD80 (B7-1) and CD86 (B7-2) on the surface of DC that interacts with CD28 and cytotoxic T lymphocyte antigen4 (CTLA4) on the surface of the T cells (Signal 2). CD28 is associated with the activation of T cells, whereas CTLA4 (CD158) is more regulatory. After signal 2, DC produces essential cytokines, which binds to receptors on T cells' cell surface and drives them to secrete different cytokines. For example, secretion of IL-10, IL12, IL-4, or a combination of IL-6/ IL-1/ IL- 23 promotes differentiation to Th1, Th2, Th3, and Th17, respectively. (Kasper & Shoemaker, 2010 and Fletcher et al., 2010).
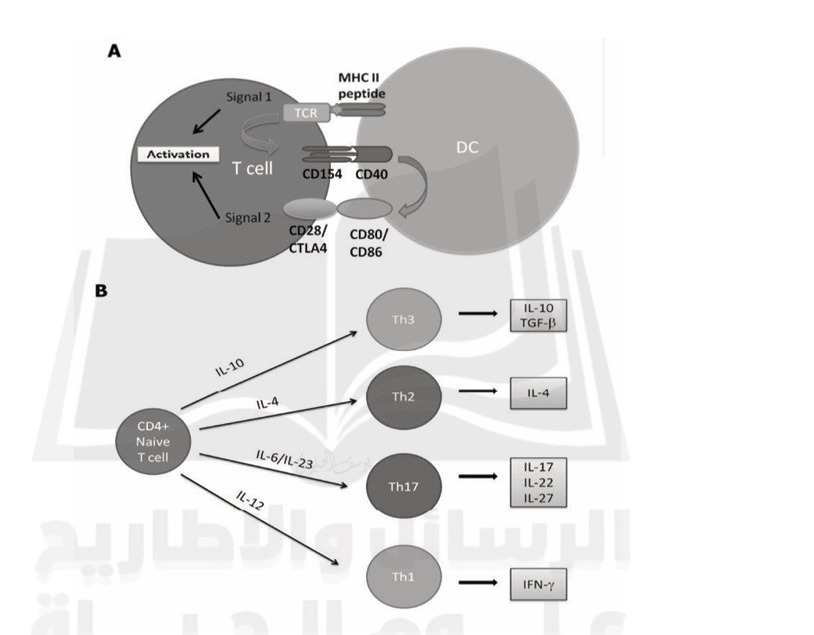
Fig1. Activation and differentiation of the naive CD4+ T cells. A. On activation, the dendritic cells (DC) interact with the T-cell receptor (TCR) on the surface of the T cells (signal 1). B. Naive CD4+ T cells differentiation depends on the cytokine milieu. (Fletcher, et al., 2010; Kasper and Shoemaker, 2010).
References
-------------
Al-Obaidi, A. B., Ali, Z. A., Rasool Almashta, S. A., and Faisel Ghazi, H. (2021). the Potential Role of Epstein Barr Virus in Multiple Sclerosis Molecular and Serological Study. Wiadomosci Lekarskie (Warsaw, Poland : 1960), 75(3). https://doi.org/10.36740/wlek202203122
Babbe, H., Roers, A., Waisman, A., Lassmann, H., Goebels, N., Hohlfeld, R., and Rajewsky, K. (2000). Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. The Journal of experimental medicine, 192(3), 393-404.
Baecher-Allan, C., Kaskow, B. J., and Weiner, H. L. (2018). Multiple sclerosis: mechanisms and immunotherapy. Neuron, 97(4), 742-768.
Bar-Or, A., and Li, R. (2021). Cellular immunology of relapsing multiple sclerosis: interactions, checks, and balances. The Lancet Neurology, 20(6), 470-483.
Dwyer, C. M. (2020). The Role of MERTK in Central Inflammatory Demyelination (Doctoral dissertation, The University of Melbourne).
Fletcher, J. M., Lalor, S. J., Sweeney, C. M., Tubridy, N., and Mills, K. H. G. (2010). T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clinical and Experimental Immunology, 162(1), 1-11.
Höftberger, R., Aboul-Enein, F., Brueck, W., Lucchinetti, C., Rodriguez, M., Schmidbauer, M., Jellinger, K., and Lassmann, H. (2004). Expression of major histocompatibility complex class l molecules on the different cell types in multiple sclerosis lesions. Brain Pathology, 14(1), 43–50.
Kasper, L. H., and Shoemaker, J. (2010). Multiple sclerosis immunology: the healthy immune system vs the MS immune system. Neurology, 74(1 Supplement 1), S2-S8.
Mexhitaj, I., Nyirenda, M. H., Li, R., O’Mahony, J., Rezk, A., Rozenberg, A., and Bar-Or, A. (2019). Abnormal effector and regulatory T cell subsets in paediatric-onset multiple sclerosis. Brain, 142(3), 617-632.
Peeters, L. M., Vanheusden, M., Somers, V., Van Wijmeersch, B., Stinissen, P., Broux, B., and Hellings, N. (2017). Cytotoxic CD4+ T cells drive multiple sclerosis progression. Frontiers in immunology, 8, 1160.
Reich, D. S., Lucchinetti, C. F., and Calabresi, P. A. (2018). Multiple Sclerosis. The New England Journal of Medicine, 378(2), 169–180. https://doi.org/10.1056/NEJMra1401483
Rodriguez, M. (2007). Effectors of demyelination and remyelination in the CNS: implications for multiple sclerosis. Brain Pathology, 17(2), 219-229.
Steinman, L. (2014). Immunology of relapse and remission in multiple sclerosis. Annual review of immunology, 32, 257-281.
Wingerchuk, D. M., and Carter, J. L. (2014). Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. In Mayo Clinic Proceedings. Elsevier. (Vol. 89, No. 2, pp. 225-240).



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|