

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Alpha Helix
المؤلف:
Peter J. Kennelly, Kathleen M. Botham, Owen P. McGuinness, Victor W. Rodwell, P. Anthony Weil
المصدر:
Harpers Illustrated Biochemistry
الجزء والصفحة:
32nd edition.p36-37
2025-02-08
1078
The polypeptide backbone of an α helix is twisted by an equal amount about each α-carbon with a phi angle of approximately −57° and a psi angle of approximately −47°. A complete turn of the helix contains an average of 3.6 aminoacyl residues, and its pitch or rise per turn is 0.54 nm (Figure 1). The R groups of each aminoacyl residue in an α helix face outward (Figure 2). Since proteins are comprised solely of L-amino acids, the only stable α helices they can form are right-handed. Schematic diagrams of proteins often represent α helices as coils or cylinders.
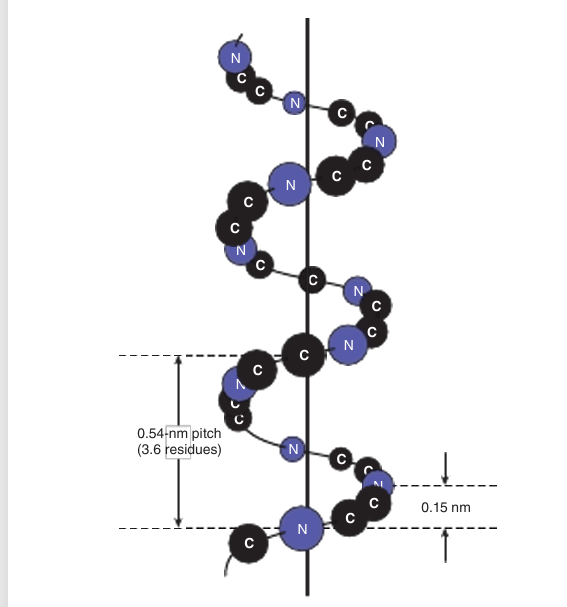
fig1. Orientation of the main chain atoms of a pep tide about the axis of an α helix.
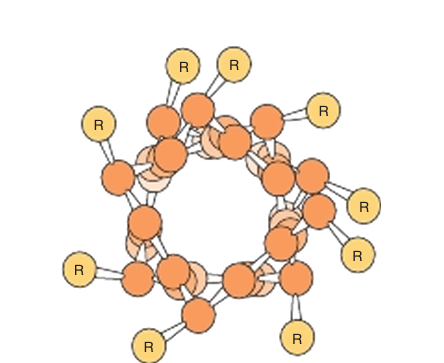
fig2. View down the axis of a polypeptide α helix .The side chains (R) are on the outside of the helix. The van der Waals radii of the atoms are larger than shown here; hence, there is almost no free space inside the helix.
The stability of an α helix arises primarily from hydrogen bonds formed between the oxygen of the peptide bond carbonyl and the hydrogen atom of the peptide bond nitrogen of the fourth residue down the polypeptide chain (Figure 3). The ability to form the maximum number of hydrogen bonds, supplemented by van der Waals interactions in the core of this tightly packed structure, provides the thermodynamic driving force for the formation of an α helix. Since the peptide bond nitrogen of proline lacks a hydrogen atom, it is incapable of forming a hydrogen bond with a carbonyl oxygen. Consequently, proline can only be stably accommodated within the first turn of an α helix. When present elsewhere, proline disrupts the conformation of the helix, producing a bend. Because it possesses such a small R group, glycine can disrupt packing, that may introduce a bend within an α helix.
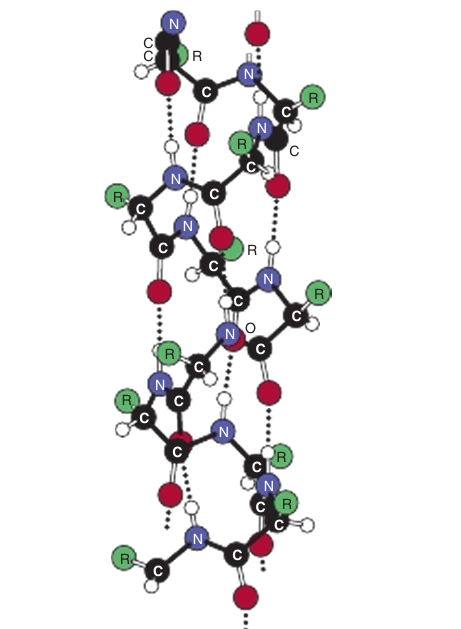
fig3. Hydrogen bonds (dotted lines) formed between H and O atoms stabilize a polypeptide in an α-helical conformation.
Many α helices have predominantly hydrophobic R groups projecting from one side along their central axis and predominantly hydrophilic R groups projecting from the other side. These amphipathic helices are well adapted to the formation of interfaces between polar and nonpolar regions such as the hydrophobic interior of a protein and its aqueous environment. Clusters of amphipathic helices can create channels, or pores, through hydrophobic cell membranes that permit specific polar molecules to pass.
 الاكثر قراءة في الكيمياء الحيوية
الاكثر قراءة في الكيمياء الحيوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















