

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Fibrin Meshwork Formation : Pathways
المؤلف:
Denise R. Ferrier
المصدر:
Lippincott Illustrated Reviews: Biochemistry
الجزء والصفحة:
6-1-2022
5591
Fibrin Meshwork Formation : Pathways
Three distinct pathways are involved in formation of the fibrin meshwork: the extrinsic pathway, the intrinsic pathway, and the common pathway. Production of FXa by the extrinsic and intrinsic pathways initiates the common pathway .
1. Extrinsic:
This pathway involves a protein, tissue factor (TF), that is not in the blood but becomes exposed when blood vessels get injured. TF (or, FIII) is a transmembrane glycoprotein abundant in vascular subendothelium. It is an extravascular accessory protein and not a protease. Any injury that exposes FIII to blood rapidly (within seconds) initiates the extrinsic (or, TF) pathway. Once exposed, TF binds a circulating Gla-containing protein, FVII, activating it through conformational change. [Note: FVII can also be activated proteolytically by thrombin .] Binding of FVII to TF requires the presence of Ca2+ and phospholipids. The TF–FVIIa complex then binds and activates FX by proteolysis (Fig. 1). Therefore, activation of FX by the extrinsic pathway occurs in association with the cell membrane. The extrinsic pathway is quickly inactivated by tissue factor pathway inhibitor (TFPI) that, in a FXa-dependent process, binds to the TF–FVIIa complex and prevents further production of FXa. [Note: TF and FVII are unique to the extrinsic pathway.]
Figure 1: The extrinsic or tissue factor (TF) pathway. Binding of FVII to exposed TF (FIII) activates FVII. [Note: The pathway is quickly inhibited by tissue factor pathway inhibitor (TFPI).] F = factor; Gla = γ-carboxyglutamate; Ca2+ = calcium; PL = phospholipid; a = active.
2. Intrinsic:
All of the protein factors involved in the intrinsic pathway are present in the blood and are, therefore, intravascular. The intrinsic pathway involves two phases: the contact phase and the FX-activation phase, each with known deficiencies.
a. Contact phase: This phase results in the activation of FXII (Hageman factor) by conformational change through binding to a negative surface. Deficiencies in FXII (or in the other proteins of this phase, high molecular weight kininogen and prekallikrein) do not result in bleeding, calling into question the importance of this phase in coagulation. However, the contact phase does play a role in inflammation. [Note: FXII can be activated proteolytically by thrombin )].
b. Factor X–activation phase: The sequence of events leading to the activation of FX to FXa by the intrinsic pathway is initiated by FXIIa (Fig. 2). FXIIa activates FXI, and FXIa activates FIX, a Glacontaining serine protease. FIXa combines with FVIIIa (a bloodborne accessory protein), and the complex activates FX, a Gla-containing serine protease. [Note: The complex containing FIXa, FVIIIa, and FX forms on exposed negatively charged membrane regions, and FX gets activated to FXa. This complex is sometimes referred to as Xase. Binding of the complex to membrane phospholipids requires Ca2+.]
Figure 2: FX-activation phase of the intrinsic pathway. [Note: von Willebrand factor (VWF) stabilizes FVIII in the circulation.] Gla = γ-carboxyglutamate; PL = phospholipid; a = active; F = factor; Ca2+ = calcium.
c. Factor XII deficiency: A deficiency in FXII does not lead to a bleeding disorder. This is because FXI, the next protein in the cascade, can be activated proteolytically by thrombin .
d. Hemophilia: Hemophilia is a coagulopathy, a defect in the ability to clot. Hemophilia A, which accounts for 80% of all hemophilia, results from deficiency of FVIII, whereas deficiency of FIX results in hemophilia B. Each deficiency is characterized by decreased and delayed ability to clot and/or formation of abnormally friable (easily disrupted) clots. This can be manifested, for example, by bleeding into the joints (Fig. 3). The extent of the factor deficiency determines the severity of the disease. Current treatment for hemophilia is factor replacement therapy using FVIII or FIX obtained from pooled human blood or from recombinant DNA technology. However, antibodies to the factors can develop. Gene therapy is a goal. Because the genes for both proteins are on the X chromosome, hemophilia is an X-linked disorder. [Note: Deficiency of FXI results in a bleeding disorder that sometimes is referred to as hemophilia C.]
Figure 3: Acute bleeding into joint spaces (hemarthrosis) in an individual with hemophilia.
The inactivation of the extrinsic pathway by TFPI results in dependence on the intrinsic pathway for continued production of FXa. This explains why individuals with hemophilia bleed even though they have an intact extrinsic pathway.
3. Common:
FXa produced by both the intrinsic and the extrinsic paths initiates the common pathway, a sequence of reactions that results in the generation of fibrin (FIa), as shown in Figure 4. FXa associates with FVa (a bloodborne accessory protein) and, in the presence of Ca2+ and phospholipids, forms a membrane-bound complex referred to as prothrombinase. The complex cleaves prothrombin (FII) to thrombin (FIIa). [Note: FVa potentiates the proteolytic activity of FXa.] The binding of Ca2+ to the Gla residues in FII facilitates the binding of FII to the membrane and to the prothrombinase complex, with subsequent cleavage to FIIa. Cleavage excises the Gla-containing region, releasing FIIa from the membrane and, thereby, freeing it to activate fibrinogen (FI) in the blood. [Note: This is the only example of cleavage of a Gla protein that results in the release of a Gla-containing peptide. The peptide travels to the liver where it is thought to act as a signal for increased production of clotting proteins.] Oral, direct inhibitors of FXa have been approved for clinical use as anticoagulants. In contrast to warfarin, they have a more rapid onset and shorter half-life and do not require routine monitoring.
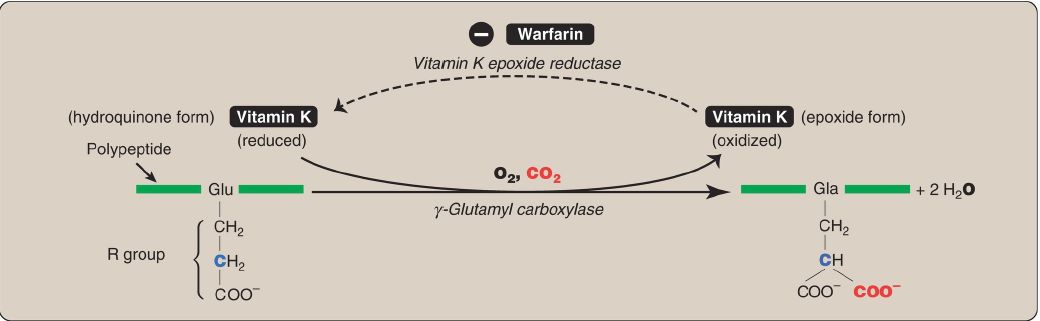
Figure 4: Generation of fibrin by FXa and the common pathway. F = factor; Gla = γ-carboxyglutamate; PL = phospholipid; a = active; Ca2+ = calcium.
A common point mutation (G20210A) in which an adenine (A) replaces a guanine (G) at nucleotide 20210 in the 3′ untranslated region of the gene for FII leads to increased levels of FII in the blood. This results in thrombophilia, a condition characterized by an increased tendency for blood to clot.
a. Fibrinogen cleavage to fibrin: FI is a soluble glycoprotein made by the liver. It consists of dimers of three different polypeptide chains [(αβγ)2] held together at the N termini by disulfide bonds. The N termini of the α and β chains form “tufts” on the central of three globular domains (Fig. 5). The tufts are negatively charged and result in repulsion between FI molecules. Thrombin (FIIa) cleaves the charged tufts (releasing fibrinopeptides A and B), and FI becomes FIa.
As a result of the loss of charge, the FIa monomers are able to noncovalently associate in a staggered array, and a soft (soluble) fibrin clot is formed.
Figure 5: Conversion of fibrinogen to fibrin and formation of the soft fibrin clot. [Note: D and E refer to nodular domains on the protein.]
b. Fibrin cross-linking: The associated FIa molecules get covalently cross-linked. This converts the soft clot to a hard (insoluble) clot. FXIIIa, a transglutaminase, covalently links the γ-carboxamide of a glutamine residue in one FIa molecule to the ε-amino of a lysine residue in another through formation of an isopeptide bond and release of ammonia (Fig. 6). [Note: FXIII is also activated by thrombin.]
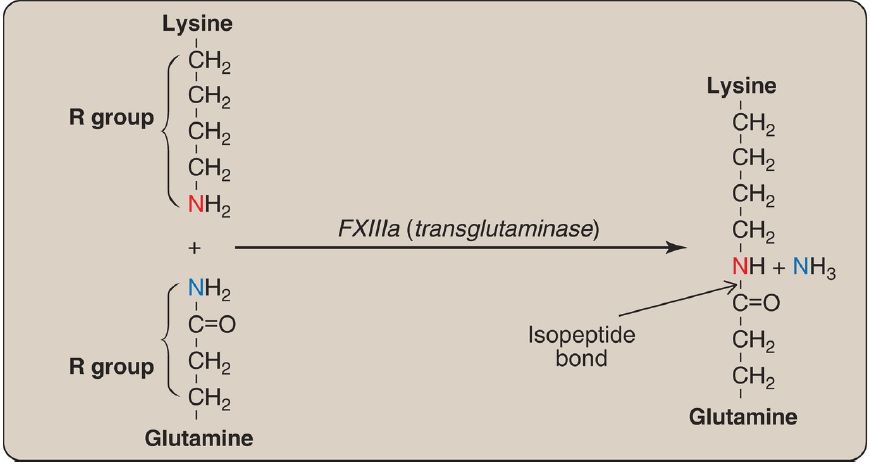
Figure 6: Cross-linking of fibrin. FXIIIa forms a covalent isopeptide bond between lysine and glutamine residues. F = factor; NH3 = ammonia.
c. Importance of thrombin: The activation of FX by the extrinsic pathway provides the “spark” of FXa that results in the initial activation of thrombin. FIIa then activates factors of the common (FV, FI, FXIII), intrinsic (FXI, FVIII), and extrinsic (FVII) pathways (Fig. 7). It also activates FXII of the contact phase. The extrinsic pathway, then, initiates clotting by the generation of FXa, and the intrinsic pathway amplifies and sustains clotting after the extrinsic pathway has been inhibited by TFPI. [Note: Hirudin, a peptide secreted from the salivary gland of medicinal leeches, is a potent direct thrombin inhibitor (DTI).
Injectable recombinant hirudin has been approved for clinical use. Dabigatran is an oral DTI.] Additional crosstalk between the pathways of clotting is achieved by the FVIIa–TF-mediated activation of the intrinsic pathway and the FXIIa-mediated activation of the extrinsic pathway. The complete picture of physiologic blood clotting via the formation of a hard fibrin clot is shown in Figure 8. The factors of the clotting cascade are shown organized by function in Figure 9.
Figure 7: The importance of thrombin in formation of the fibrin clot. a = active; F = factor.
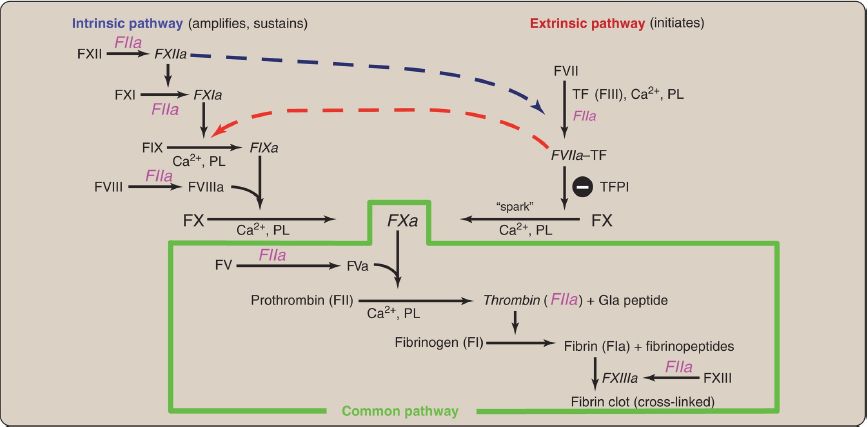
Figure 8: The complete picture of physiologic blood clotting via the formation of a cross-linked (hard) fibrin clot. a = active; F = factor; TF = tissue factor; TFPI = tissue factor pathway inhibitor; PL = phospholipid; Ca2+ = calcium; Gla = γ-carboxyglutamate.
Figure 9: Protein factors of the clotting cascade organized by function. The activated form would be denoted by an “a” after the numeral. [Note: Calcium is IV. There is no VI. I (fibrin) is neither a protease nor an accessory protein. XIII is a transglutaminase.] Gla = γ-carboxyglutamate.
Clinical laboratory tests are available to evaluate the extrinsic through common pathways (prothrombin time [PT] using thromboplastin and expressed as the international normalized ratio [INR]) and the intrinsic through common pathways (activated partial thromboplastin time [aPTT]).
Thromboplastin is a combination of phospholipids + FIII. A derivative, partial thromboplastin contains just the phospholipid portion because FIII is not needed to activate the intrinsic pathway.
 الاكثر قراءة في الكيمياء الحيوية
الاكثر قراءة في الكيمياء الحيوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















