


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 12-5-2016
Date: 8-10-2018
Date: 23-5-2017
|
The strain in ring compounds can be evaluated quantitatively by comparing the heats of combustion per CH2 group, as in Table 12-3. The data indicate that cyclohexane is virtually strain-free, because the heat of combustion per CH2 is the same as for alkanes (157.4kcal mol−1). The increase for the smaller rings clearly reflects increasing angle strain and, to some extent, unfavorable interactions between nonbonded atoms. For rings from C7 to C12 there appears to be a residual strain for each additional CH2 of 1 to 1.5kcal mol−1. These rings can be puckered into flexible conformations with normal C−C−C angles, from C7 to C13 such arrangements all have pairs of partially eclipsed or interfering hydrogens. The larger cycloalkanes such as cyclopentadecane appear to be essentially strain-free.
We expect that the total strain in cycloalkanes of the type (CH2)n should decrease rapidly in the order n=2>n=3>n=4. However, the data of Table 12-3 show that the order actually is 3≅4>2. This difference in order often is disguised by dividing the heats of combustion by the numbers of CH2 groups and showing that the heats of combustion per CH2 are at least in the order expected from bond-angle strain. This stratagem does not really solve the problem.
It is important to recognize that when we evaluate strain from the heats of combustion per CH2 group, we are assuming that the C−H bonds have the same strength, independent of nn. However, the bond-dissociation energies of each of the C−H bonds of ethene and cyclopropane are greater than of the C2−H bonds of propane (Table 4-6).
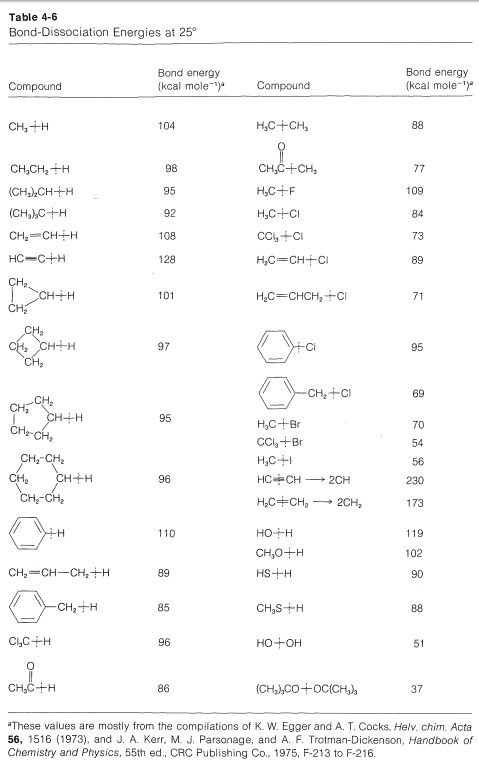
Any amount that these bonds are stronger than normal will make the strain energies judged from heats of combustion appear to be less. If we take the C−H bonds to be on average 2kcal mol−12kcal mol−1 stronger in cyclobutane, 6kcal mol−1 stronger in cyclopropane, and 13kcal mol−113kcal mol−1 in ethene, we can correct the carbon-carbon strain energies accordingly. For cyclobutane the corrected strain then is 8×2 (for the eight C−H bonds) +26.3 (total strain from Table 12-3) =42.3kcal mol−1. The corresponding figures for cyclopropane are 6×6+27.6=63.6kcal mol−1, and for ethene, 4×13+22.4=74.4kcal mol−1. The results support the intuitive expectations by giving larger differences in the right direction for the strain energies of cyclobutane, cyclopropane, and ethene. Whether this analysis is quantitatively correct or not, it does give some indication of why strain energy is not a very precise concept - unless we can reliably estimate the net effects of a strain.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|