


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-7-2016
Date: 27-8-2018
Date: 28-8-2018
|
The exact mechanisms of heterogeneous reactions are difficult to determine, but much interesting and helpful information has been obtained for catalytic hydrogenation. The metal catalyst is believed to act by binding the reactants at the surface of a crystal lattice. As an example, consider the surface of a nickel crystal (Figure 11-1). The nickel atoms at the surface have fewer neighbors (lower covalency) than the atoms in the interior of the crystal. The surface atoms therefore have residual bonding capacity and might be expected to combine with a variety of substances.
Figure 11-1: Left: Schematic representation of a nickel crystal in cross section showing residual valences at the surface atoms. Right: Adsorption of ethene on the surface of the nickel crystal with formation of C−Ni bonds.
It has been shown experimentally that ethene combines exothermically (ΔH0=−60kcal/mol) and reversibly with a metal surface. Although the precise structure of the ethene-nickel complex is unknown, the bonding to nickel must involve the electrons of the double bond because saturated hydrocarbons, such as ethane, combine only weakly with the nickel surface. A possible structure with carbon-nickel σσ bonds is shown in Figure 11-1.
Hydrogen gas combines with nickel quite readily with dissociation of the H−H bonds and formation of Ni−H bonds (nickel hydride bonds). The overall hydrogenation process is viewed as a series of reversible and sequential steps, as summarized in Figure 11-2. First the reactants, hydrogen and ethene, are adsorbed on the surface of the metal catalyst. The energies of the metal-hydrogen and metal-carbon bonds are such that, in a second step, a hydrogen is transferred to carbon to give an ethyl attached to nickel. This is the halfway point. In the next step, the nickel-carbon bond is broken and the second carbon-hydrogen bond is formed. Hydrogenation is now complete and the product is desorbed from the catalyst surface.
Figure 11-2: A possible cycle of reactions for catalytic hydrogenation of ethene. Ethane is held much less tightly than ethene on the catalyst surface, so as long as ethene is present no significant amount of ethane is bound.
Ethane has a low affinity for the metal surface and, when desorbed, creates a vacant space for the adsorption of new ethene and hydrogen molecules. The cycle continues until one of the reagents is consumed or some material is adsorbed that "poisons" the surface and makes it incapable of further catalytic activity. Because the reaction occurs only on the surface, small amounts of a catalyst poison can completely stop the reaction.
As might be expected for the postulated mechanism, the spacings of the metal atoms in the crystal lattice are quite important in determining the hydrogenation rates. The mechanism also accounts for the observation that hydrogen usually adds to an alkene in the suprafacial manner. To illustrate, 1,2-dimethylcyclohexene is reduced to cis-1,2-dimethylcyclohexane:
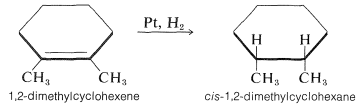



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|