


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 19-1-2022
التاريخ: 27-8-2018
التاريخ: 28-8-2018
التاريخ: 28-8-2018
|
The alkynes behave in many ways as if they were doubly unsaturated alkenes. For example, bromine adds to ethyne in two stages - first to give trans-1,2-dibromoethene by antarafacial addition, and finally to give 1,1,2,2-tetrabromoethane:
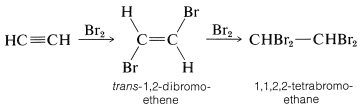
Likewise, anhydrous hydrogen fluoride adds first to give fluoroethene and ultimately to give 1,1-difluoroethane:
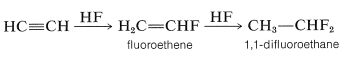
However, there is an interesting contrast in reactivity. Alkynes are substantially less reactive than corresponding alkenes toward many electrophiles. This is perhaps surprising because the electrons of a triple bond, like those of a double bond, are highly exposed, which suggests that the reactivity (nucleophilicity) of a triple bond should be high. Evidently this is not the case. A simple but reasonable explanation is that the carbocation formed from the alkyne is less stable than that from the alkene because it cannot achieve the sp2 hybrid-orbital configuration expected to be the most stable arrangement for a carbocation:
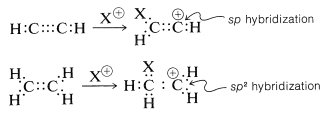



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|