


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 30-9-2020
Date: 27-8-2018
Date: 30-9-2020
|
The reactions of alkanes are homolytic processes, which means that the bonds are made and broken through radical or atomic intermediates. In contrast, the SN and E reactions of alkyl halides involve heterolytic bond cleavage and ionic reagents or products. An especially important factor contributing to the differences between the reactions of the alkanes and alkyl halides is the slight ionic character of C−H compared to C-halide bonds . The alkenes are like the alkanes in being nonpolar compounds and it may come as a surprise that many important reactions of alkenes are heterolytic reactions. Why should this be so? No doubt because the electrons in the alkene double bonds are more exposed and accessible than the electrons in an alkane C−C bond.
The electrons of the double bond are pushed outward by their mutual repulsions, and their average positions are considerably farther from the bond axis than the electron positions of a single bond (Figure 10-6). In such circumstances, electrophilic reagents, which act to acquire electrons in chemical reactions, are expected to be particularly reactive. This is actually the case. Furthermore, reagents that are primarily nucleophilic (electron-donating) are notoriously poor for initiating reactions at carbon-carbon double bonds. Exceptions occur when the double bonds carry substituents with a sufficiently high degree of electron-attracting power to reduce the electron density in the double bond enough to permit attack by a nucleophilic agent.
Figure 10-6: Schematic representations of average densities of electrons in carbon-carbon single and double bonds.
Example of electrophilic reagents that normally add to carbon-carbon double bonds of alkenes to give saturated compounds include halogens (Cl2, Br2, and I2), hydrogen halides (HCl and HBr), hypohalous acids (HOCl and HOBr), water, and sulfuric acid:
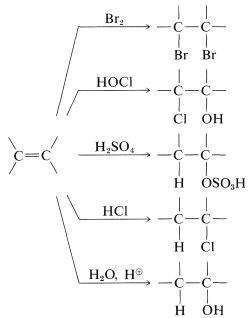
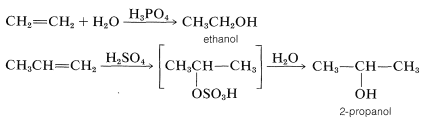
The mechanisms of these reactions have much in common and have been studied extensively from this point of view. They also have very considerable synthetic utility. The addition of water to alkenes (hydration) is particularly important for the preparation of a number of commercially important alcohols. Thus ethanol and 2-propanol (isopropyl alcohol) are made on a very large scale by the hydration of the corresponding alkenes (ethene and propene) using sulfuric or phosphoric acids as catalysts. The nature of this type of reaction will be described later.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|